
Chemistry: A Molecular Approach (4th Edition)
4th Edition
ISBN: 9780134112831
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22, Problem 32E
Interpretation Introduction
Interpretation: The molecule is a lipid, state the kind of lipid if it is the fatty acid or triglyceridelassify it as saturated or unsaturated. Whether or not each molecule is a lipid.
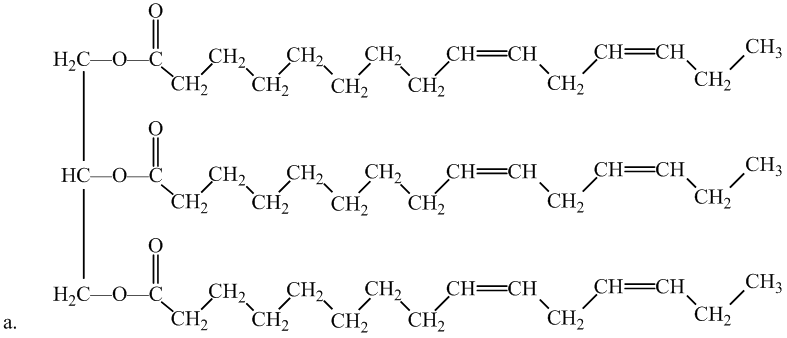
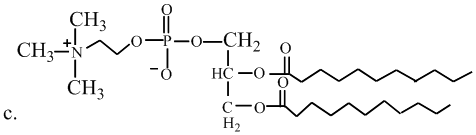
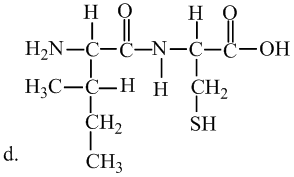
Concept Introduction:
Fats and oils are triglycerides, triesters composed of glycerol with three fatty acids attached. Unsaturated fats consist of at least one double bond in the main carbon chain of the molecule, whereas saturated fatty acids contain no double bond in the main carbon chain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
8:17 PM Sun Mar 30
Draw the major product of this reaction. Ignore
inorganic byproducts.
HSCH2CH2CH2SH, BF3
Probler
Drawing
Ato
Bonds
Cl
presented by Mr L
How the coprion. (Il Done in no wraction, dew the starting redential)
до
8:16 PM Sun Mar 30
K
Draw the major product of this reaction. Ignore
inorganic byproducts.
Proble
1. CH3MgBr
2. H3O+
F
Drawing
Chapter 22 Solutions
Chemistry: A Molecular Approach (4th Edition)
Ch. 22 - Prob. 1SAQCh. 22 - Prob. 2SAQCh. 22 - Prob. 3SAQCh. 22 - Prob. 4SAQCh. 22 - Prob. 5SAQCh. 22 - Prob. 6SAQCh. 22 - Prob. 7SAQCh. 22 - Prob. 8SAQCh. 22 - Q9. Peptide bonds occur in which type of...Ch. 22 - Q10. How many nucleotides are required to code for...
Ch. 22 - 1. What is biochemistry? What significant advances...Ch. 22 - Prob. 2ECh. 22 - Prob. 3ECh. 22 - 4. What effect do double bonds have within the...Ch. 22 - Prob. 5ECh. 22 - Prob. 6ECh. 22 - 7. Describe the basic structure of phospholipids...Ch. 22 - 8. What is a steroid? List some functions of...Ch. 22 - Prob. 9ECh. 22 - Prob. 10ECh. 22 - Prob. 11ECh. 22 - Prob. 12ECh. 22 - Prob. 13ECh. 22 - Prob. 14ECh. 22 - Prob. 15ECh. 22 - Prob. 16ECh. 22 - Prob. 17ECh. 22 - Prob. 18ECh. 22 - Prob. 19ECh. 22 - Prob. 20ECh. 22 - Prob. 21ECh. 22 - Prob. 22ECh. 22 - Prob. 23ECh. 22 - Prob. 24ECh. 22 - Prob. 25ECh. 22 - Prob. 26ECh. 22 - 27. What is a codon? A gene? A chromosome?
Ch. 22 - Prob. 28ECh. 22 - Prob. 29ECh. 22 - Prob. 30ECh. 22 - Prob. 31ECh. 22 - Prob. 32ECh. 22 - Prob. 33ECh. 22 - Prob. 34ECh. 22 - 35. Draw structures showing the reaction of...Ch. 22 - Prob. 36ECh. 22 - Prob. 37ECh. 22 - Prob. 38ECh. 22 - Prob. 39ECh. 22 - Prob. 40ECh. 22 - Prob. 41ECh. 22 - Prob. 42ECh. 22 - Prob. 43ECh. 22 - Prob. 44ECh. 22 - Prob. 45ECh. 22 - Prob. 46ECh. 22 - Prob. 47ECh. 22 - Prob. 48ECh. 22 - 49. Draw each amino acid in its dipolar ion...Ch. 22 - 50. Draw each amino acid in its dipolar ion...Ch. 22 - Prob. 51ECh. 22 - Prob. 52ECh. 22 - Prob. 53ECh. 22 - Prob. 54ECh. 22 - Prob. 55ECh. 22 - Prob. 56ECh. 22 - Prob. 57ECh. 22 - Prob. 58ECh. 22 - 59. A phenylalanine amino acid on a protein strand...Ch. 22 - 60. An amino acid on a protein strand forms a...Ch. 22 -
61. The amino acid sequence in one section of a...Ch. 22 - Prob. 62ECh. 22 - Prob. 63ECh. 22 - Prob. 64ECh. 22 - Prob. 65ECh. 22 - Prob. 66ECh. 22 - Prob. 67ECh. 22 - Prob. 68ECh. 22 - Prob. 69ECh. 22 - Prob. 70ECh. 22 - 71. Determine the class of biochemical compound...Ch. 22 - Prob. 72ECh. 22 - 73. What is the difference between a codon and a...Ch. 22 - 74. What is the difference between a fatty acid...Ch. 22 - Prob. 75ECh. 22 - Prob. 76ECh. 22 - Prob. 77ECh. 22 - Prob. 78ECh. 22 - 79. Determining the amino acid sequence in a...Ch. 22 - Prob. 80ECh. 22 - Prob. 81ECh. 22 - 82. Calculate the mass percent of phosphorus in a...Ch. 22 - 83. The double helical structure of DNA disrupts...Ch. 22 - Prob. 84ECh. 22 - Prob. 85ECh. 22 - Prob. 86ECh. 22 - Prob. 87ECh. 22 - Prob. 88ECh. 22 - 89. Write the major equilibrium that is...Ch. 22 - Prob. 90ECh. 22 - Prob. 91ECh. 22 - 92. The genetic code is random, which means that a...Ch. 22 - Prob. 93QGWCh. 22 - Prob. 94QGWCh. 22 - Prob. 95QGWCh. 22 - Prob. 96QGWCh. 22 - Prob. 97QGWCh. 22 - Prob. 98DIA
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forwardPredict the major organic product(s) for the following reactions.arrow_forward
- Provide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY