
ORGANIC CHEMISTRY 1 TERM ACCESS
3rd Edition
ISBN: 9781119661511
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 21.6, Problem 40CC
Interpretation Introduction
Interpretation:
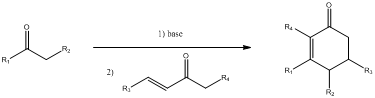
Mechanism for the given transformation has to be entered.
Concept introduction:
Robinson annulation is used for synthesizing polycyclic compound, namely six membered ring. This method is used to convert
- Michael addition
- Aldol condensation.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify the starting material in the following reaction. Click the "draw structure" button to launch the
drawing utility.
draw structure ...
[1] 0 3
C10H18
[2] CH3SCH3
H
In an equilibrium mixture of the formation of ammonia from nitrogen and hydrogen, it is found that
PNH3 = 0.147 atm, PN2 = 1.41 atm and Pн2 = 6.00 atm. Evaluate Kp and Kc at 500 °C.
2 NH3 (g) N2 (g) + 3 H₂ (g)
K₂ = (PN2)(PH2)³ = (1.41) (6.00)³ = 1.41 x 104
What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the
"draw structure" button to launch the drawing utility.
and two equivalents of CH2=O
draw structure ...
Chapter 21 Solutions
ORGANIC CHEMISTRY 1 TERM ACCESS
Ch. 21.1 - Prob. 1CCCh. 21.1 - Prob. 2CCCh. 21.1 - Prob. 3CCCh. 21.1 - Prob. 1LTSCh. 21.1 - Prob. 4PTSCh. 21.1 - APPLY the skill
As with aldehydes and ketones, the...Ch. 21.1 - Draw the enolate ion that is formed when each of...Ch. 21.1 - Prob. 7CCCh. 21.2 - Prob. 8CCCh. 21.2 - Prob. 9CC
Ch. 21.2 - Prob. 10CCCh. 21.2 - Prob. 11CCCh. 21.2 - Prob. 12CCCh. 21.2 - Prob. 13CCCh. 21.3 - Prob. 2LTSCh. 21.3 - Prob. 14PTSCh. 21.3 - Prob. 15PTSCh. 21.3 - Prob. 16ATSCh. 21.3 - Prob. 3LTSCh. 21.3 - Prob. 17PTSCh. 21.3 - Prob. 18ATSCh. 21.3 - Prob. 4LTSCh. 21.3 - Prob. 19PTSCh. 21.3 - Prob. 20ATSCh. 21.3 - Prob. 21CCCh. 21.3 - Prob. 22CCCh. 21.3 - Prob. 23CCCh. 21.4 - Prob. 24CCCh. 21.4 - Prob. 25CCCh. 21.4 - Prob. 26CCCh. 21.4 - Prob. 27CCCh. 21.4 - Prob. 28CCCh. 21.5 - Prob. 29CCCh. 21.5 - Prob. 30CCCh. 21.5 - Prob. 5LTSCh. 21.5 - Prob. 31PTSCh. 21.5 - Prob. 32ATSCh. 21.5 - Prob. 6LTSCh. 21.5 - Prob. 33PTSCh. 21.5 - Prob. 34ATSCh. 21.6 - Prob. 35CCCh. 21.6 - Prob. 36CCCh. 21.6 - Prob. 37CCCh. 21.6 - Prob. 7LTSCh. 21.6 - Prob. 38PTSCh. 21.6 - Prob. 39ATSCh. 21.6 - Prob. 40CCCh. 21.6 - Prob. 41CCCh. 21.7 - Prob. 8LTSCh. 21.7 - Prob. 42PTSCh. 21.7 - Prob. 43PTSCh. 21.7 - Prob. 9LTSCh. 21.7 - Prob. 45PTSCh. 21.7 - Prob. 46ATSCh. 21 - Prob. 47PPCh. 21 - Prob. 48PPCh. 21 - Prob. 49PPCh. 21 - Prob. 50PPCh. 21 - Prob. 51PPCh. 21 - Prob. 52PPCh. 21 - Prob. 53PPCh. 21 - Prob. 54PPCh. 21 - Prob. 55PPCh. 21 - Prob. 56PPCh. 21 - Prob. 57PPCh. 21 - Prob. 58PPCh. 21 - Prob. 59PPCh. 21 - Prob. 60PPCh. 21 - Prob. 61PPCh. 21 - Prob. 62PPCh. 21 - Prob. 63PPCh. 21 - Prob. 64PPCh. 21 - Prob. 65PPCh. 21 - Prob. 66PPCh. 21 - Prob. 67PPCh. 21 - Prob. 68PPCh. 21 - Prob. 69PPCh. 21 - Prob. 70PPCh. 21 - Prob. 71PPCh. 21 - Prob. 72PPCh. 21 - Prob. 73PPCh. 21 - Prob. 74PPCh. 21 - Prob. 75PPCh. 21 - Prob. 76PPCh. 21 - Prob. 77PPCh. 21 - Prob. 78PPCh. 21 - Prob. 79PPCh. 21 - Prob. 80PPCh. 21 - Prob. 81PPCh. 21 - Prob. 82PPCh. 21 - Prob. 83PPCh. 21 - Prob. 84PPCh. 21 - Prob. 85PPCh. 21 - Prob. 86PPCh. 21 - Prob. 87PPCh. 21 - Prob. 88PPCh. 21 - Prob. 89IPCh. 21 - Prob. 90IPCh. 21 - Prob. 91IPCh. 21 - Prob. 92IPCh. 21 - Prob. 93IPCh. 21 - Prob. 94IPCh. 21 - Prob. 95IPCh. 21 - Prob. 96IPCh. 21 - Prob. 97IPCh. 21 - Prob. 98IPCh. 21 - Prob. 99IPCh. 21 - Prob. 100IPCh. 21 - Prob. 101IPCh. 21 - Prob. 102IPCh. 21 - Prob. 103IPCh. 21 - Prob. 104IPCh. 21 - Prob. 105IPCh. 21 - Prob. 106IPCh. 21 - Prob. 107IPCh. 21 - Prob. 108IPCh. 21 - Prob. 109IPCh. 21 - Prob. 110IPCh. 21 - Prob. 111IPCh. 21 - Prob. 112IPCh. 21 - Prob. 113IPCh. 21 - Prob. 114IPCh. 21 - Prob. 115IPCh. 21 - Prob. 116CPCh. 21 - Prob. 117CPCh. 21 - Prob. 118CP
Knowledge Booster
Similar questions
- H-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forwardDraw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY