
Chemistry: AP Edition - Package
9th Edition
ISBN: 9781285729473
Author: ZUMDAHL
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 97CP
Sketch and explain the most likely crystal field diagram for the following complex ion:
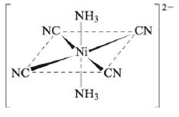
Note: The CN– ligand produces a much stronger crystal field than NH3. Assume the NH3 ligands lie on the z axis.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Explain why the S-F bond strength is 367 kJ/mol in SF2 and 329 kJ/mol in SF6.
Would Si(CH3)3F react with AgCl? If so, write out the balanced chemical equation. If not,explain why no reaction would take place.
NH3 reacts with boron halides (BX3 where X = F, Cl, Br, or I) to form H3N-BX3 complexes.Which of these complexes will have the strongest N-B bond? Justify your answer
Chapter 21 Solutions
Chemistry: AP Edition - Package
Ch. 21 - What two first-row transition metals have...Ch. 21 - Prob. 2RQCh. 21 - Prob. 3RQCh. 21 - Prob. 4RQCh. 21 - Prob. 5RQCh. 21 - Prob. 6RQCh. 21 - Prob. 7RQCh. 21 - Prob. 8RQCh. 21 - Prob. 9RQCh. 21 - Prob. 10RQ
Ch. 21 - Prob. 1ALQCh. 21 - Prob. 2ALQCh. 21 - Prob. 3ALQCh. 21 - Prob. 4ALQCh. 21 - Prob. 5QCh. 21 - Four different octahedral chromium coordination...Ch. 21 - Prob. 7QCh. 21 - Prob. 8QCh. 21 - Prob. 9QCh. 21 - Prob. 10QCh. 21 - Prob. 11QCh. 21 - Prob. 12QCh. 21 - Prob. 13QCh. 21 - Prob. 14QCh. 21 - Which of the following ligands are capable of...Ch. 21 - Prob. 16QCh. 21 - Prob. 17QCh. 21 - Prob. 18QCh. 21 - Prob. 19QCh. 21 - Prob. 20QCh. 21 - Prob. 21ECh. 21 - Prob. 22ECh. 21 - Prob. 23ECh. 21 - Prob. 24ECh. 21 - Prob. 25ECh. 21 - Prob. 26ECh. 21 - Prob. 27ECh. 21 - Prob. 28ECh. 21 - Prob. 29ECh. 21 - When an aqueous solution of KCN is added to a...Ch. 21 - Prob. 31ECh. 21 - A coordination compound of cobalt(III) contains...Ch. 21 - Prob. 33ECh. 21 - Prob. 34ECh. 21 - Prob. 35ECh. 21 - Prob. 36ECh. 21 - Prob. 37ECh. 21 - Give formulas for the following complex ions. a....Ch. 21 - Prob. 39ECh. 21 - Prob. 40ECh. 21 - Prob. 41ECh. 21 - Amino acids can act as ligands toward transition...Ch. 21 - Prob. 43ECh. 21 - Prob. 44ECh. 21 - Prob. 45ECh. 21 - Prob. 46ECh. 21 - Prob. 47ECh. 21 - Prob. 48ECh. 21 - Prob. 49ECh. 21 - Prob. 50ECh. 21 - Prob. 51ECh. 21 - Prob. 52ECh. 21 - The CrF64 ion is known to have four unpaired...Ch. 21 - Prob. 54ECh. 21 - Prob. 55ECh. 21 - The complex ion Fe(CN)63 is paramagnetic with one...Ch. 21 - Prob. 57ECh. 21 - Prob. 58ECh. 21 - Prob. 59ECh. 21 - Prob. 60ECh. 21 - The wavelength of absorbed electromagnetic...Ch. 21 - The complex ion NiCL42 has two unpaired electrons,...Ch. 21 - How many unpaired electrons are present in the...Ch. 21 - The complex ion PdCl42is diamagnetic. Propose a...Ch. 21 - Prob. 65ECh. 21 - Prob. 66ECh. 21 - Prob. 67ECh. 21 - Prob. 68ECh. 21 - Silver is sometimes found in nature as large...Ch. 21 - Prob. 70ECh. 21 - Prob. 71AECh. 21 - The compound cisplatin, Pt(NH3)2Cl2, has been...Ch. 21 - Prob. 73AECh. 21 - Prob. 74AECh. 21 - Prob. 75AECh. 21 - Prob. 76AECh. 21 - Prob. 77AECh. 21 - Name the following coordination compounds. a....Ch. 21 - Prob. 79AECh. 21 - Prob. 80AECh. 21 - Prob. 81AECh. 21 - Prob. 82AECh. 21 - Prob. 83CWPCh. 21 - Which of the following molecules exhibit(s)...Ch. 21 - Prob. 85CWPCh. 21 - The following table indicates the number of...Ch. 21 - Prob. 87CWPCh. 21 - Which of the following statement(s) is( are) true?...Ch. 21 - Consider the following complex ion, where A and B...Ch. 21 - Consider the pseudo-octahedral complex ion of...Ch. 21 - Prob. 91CPCh. 21 - Prob. 92CPCh. 21 - Chelating ligands often form more stable complex...Ch. 21 - Prob. 94CPCh. 21 - Prob. 95CPCh. 21 - Prob. 96CPCh. 21 - Sketch and explain the most likely crystal field...Ch. 21 - The ferrate ion, FeO42, is such a powerful...Ch. 21 - Ammonia and potassium iodide solutions are added...Ch. 21 - a. In the absorption spectrum of the complex ion...Ch. 21 - There are three salts that contain complex ions of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Synthesize the following:arrow_forwardDid you report your data to the correct number of significant figures? Temperature of cold water (°C) 4.0 Temperature of hot water ("C) 87.0 Volume of cold water (mL) 94.0 Volume of hot water (mL) 78.0 Final temperature after mixing ("C) 41.0 Mass of cold water (g) 94.0 Mass of hot water (g) 78.0 Calorimeter constant (J/°C) 12.44 How to calculate the calorimeter constantarrow_forwardplease draw the arrowsarrow_forward
- Part 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forwardcan you please give the answer for both these pictures. thankyouarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY