
HUMAN BIOLOGY-EBOOK ACCESS (180 DAY)
16th Edition
ISBN: 9781260918410
Author: Mader
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2.1, Problem 4LO
Summary Introduction
To distinguish:
In-between ionic and covalent bonds.
Introduction:
The atoms like sodium and chloride form ionic bond when the electrons in the outer shell give and take electrons to achieve a stable state of eight electrons in the valence shell of the atom. The sodium atom in an ionized state has a positive charge and the chlorine atom in an ionized state has a negative charge. The covalent bond is formed by the sharing of the electrons in the outer shell orbital of the atoms. The electrons are stable in this state.
Pictorial representation:
The ionic and covalent bonding is well represented in the Fig.1.
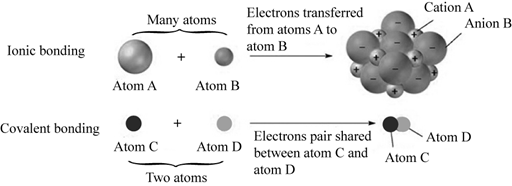
Fig.1: Ionic bonding and Covalent bonding
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain down bellow what happens to the cell:
Decreased pH in mitochondria
Increased ATP
Decreased pH in cytosol
Increased hydrolysis
Decreasing glycogen and triglycerides
Increased MAP kinase activity
Poor ion transport → For each one:→ What normally happens?→ What is wrong now?→ How does it mess up the cell?
An 1100 pound equine patient was given 20 mg/kg sucralfate 3 times a day, 2.8 mg/kg famotidine twice a day, and 10mg/kg doxycycline twice a day. Sucralfate comes as a 1 gm tablet, famotidine as 20 mg tablets, and doxycycline as 100mg tablets. All are in bottles of 100 tablets.How many total mg are needed for the patient and how many tablets of each would be needed to provide each dose?How many bottles of each would be needed to have available if this patient were to be on this drug regimen for 5 days?
The patient needs a solution of 2.5% dextrose in Lactated Ringer’s solution to run at 75 ml/hr for at least the next 12hours. LRS comes in fluid bags of 500 ml, 1 Liter, 3 Liters and 5 Liters. How can a 2.5% solution be made by adding50% dextrose to the LRS?
Chapter 2 Solutions
HUMAN BIOLOGY-EBOOK ACCESS (180 DAY)
Ch. 2.1 - Prob. 1LOCh. 2.1 - 2. Describe the structure of an atom.
Ch. 2.1 - Prob. 3LOCh. 2.1 - 4. Distinguish between ionic and covalent bonds.
Ch. 2.1 - List the number of electrons, neutrons, and...Ch. 2.1 - Prob. 2CYPCh. 2.1 - Explain the beneficial uses of radioisotopes.Ch. 2.1 - Summarize the differences between ionic and...Ch. 2.2 - Describe the properties of water.Ch. 2.2 - Prob. 2LO
Ch. 2.2 - Prob. 3LOCh. 2.2 - List the characteristics of water and explain how...Ch. 2.2 - Prob. 2CYPCh. 2.2 - Prob. 3CYPCh. 2.3 - List the four classes of organic molecules that...Ch. 2.3 - Describe the processes by which the organic...Ch. 2.3 - Prob. 1CYPCh. 2.3 - List the four classes of organic molecules.Ch. 2.3 - Prob. 3CYPCh. 2.4 - Prob. 1LOCh. 2.4 - Prob. 2LOCh. 2.4 - Prob. 3LOCh. 2.4 - Prob. 4LOCh. 2.4 - Prob. 1BTHCh. 2.4 - Prob. 2BTHCh. 2.4 - Explain the differences between a monosaccharide,...Ch. 2.4 - Prob. 2CYPCh. 2.4 - Prob. 3CYPCh. 2.5 - Prob. 1LOCh. 2.5 - Prob. 2LOCh. 2.5 - Why might physicians resort to calling HDLs and...Ch. 2.5 - Prob. 1.2BTHCh. 2.5 - Prob. 2.1BTHCh. 2.5 - Prob. 2.2BTHCh. 2.5 - Prob. 1CYPCh. 2.5 - Prob. 2CYPCh. 2.5 - Prob. 3CYPCh. 2.6 - Prob. 1LOCh. 2.6 - Prob. 2LOCh. 2.6 - List the four levels of protein structure and...Ch. 2.6 - Prob. 1CYPCh. 2.6 - Prob. 2CYPCh. 2.6 - Prob. 3CYPCh. 2.7 - Prob. 1LOCh. 2.7 - Prob. 2LOCh. 2.7 - Prob. 1CYPCh. 2.7 - Prob. 2CYPCh. 2.7 - Prob. 3CYPCh. 2 - Prob. 1ACh. 2 - Prob. 2ACh. 2 - Prob. 3ACh. 2 - Prob. 4ACh. 2 - Prob. 5ACh. 2 - Prob. 6ACh. 2 - Prob. 7ACh. 2 - Prob. 8ACh. 2 - Prob. 9ACh. 2 - Prob. 10ACh. 2 - Prob. 11ACh. 2 - Prob. 12ACh. 2 - Prob. 13ACh. 2 - Prob. 14ACh. 2 - Prob. 15ACh. 2 - Prob. 16ACh. 2 - Prob. 17ACh. 2 - Prob. 18ACh. 2 - Prob. 19ACh. 2 - This nucleic acid is typically involved in energy...Ch. 2 - Prob. 1TCCh. 2 - Prob. 2TCCh. 2 - Name another substance that is needed in the body...Ch. 2 - Prob. 4TCCh. 2 - Prob. 5TC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- “Gretchen” was a 68-pound canine who came to the VMTH as small animal surgery patient. She receivedacepromazine, 0.2 mg/kg from a 10 mg/ml solution and oxymorphone, 0.08 mg/kg from a 1 mg/ml solution before surgery.What are the mechanisms of action of acepromazine and oxymorphone? Why would they be given together?How many mg provide each dose and how many ml of each of these solutions were given?arrow_forwardAfter surgery, “Gretchen” was put on carprofen, 1 mg/pound bid (twice a day). The tablets come in 25, 75 and 100 mgsizes. Which size tablet would be appropriate?What is the mechanism of action of carprofen?An outpatient prescription was written for her so she would have enough for 10 days. How many tablets did she need?What information needs to be on her out-patient prescription?arrow_forwardJoden Koepp olor in chickens is due to incomplete dominance. BB = Black chicken, WW = White BLOOD TYPES Arhite chicken is In humans, Rh positive blood is dominant (R) over Rh negative blood (r). A man with type 0, Rh positive blood (whose mother had Rh negative blood), marries a woman with type AB, Rh negative blood. Several children were born. is? R R Genotypes Phenotypes RRR RR Rr Rr 4/16 RR R RR RK Rr Rr 4/16 rr 3/4 Rh posi 1/4 Rh negu 1/2 Rr rr rr rrrr 88 888 75 e genotype of the man? the genotype of the woman? The mother of the man had type AB blood.arrow_forward
- Please indentify the unknown organismarrow_forward5G JA ATTC 3 3 CTIA A1G5 5 GAAT I I3 3 CTIA AA5 Fig. 5-3: The Eco restriction site (left) would be cleaved at the locations indicated by the arrows. However, a SNP in the position shown in gray (right) would prevent cleavage at this site by EcoRI One of the SNPs in B. rapa is found within the Park14 locus and can be detected by RFLP analysis. The CT polymorphism is found in the intron of the Bra013780 gene found on Chromosome 1. The Park14 allele with the "C" in the SNP has two EcoRI sites and thus is cleaved twice by EcoRI If there is a "T" in that SNP, one of the EcoRI sites is altered, so the Park14 allele with the T in the SNP has only one EcoRI site (Fig. 5-3). Park14 allele with SNP(C) Park14 allele with SNPT) 839 EcoRI 1101 EcoRI 839 EcoRI Fig. 5.4: Schematic restriction maps of the two different Park14 alleles (1316 bp long) of B. rapa. Where on these maps is the CT SNP located? 90 The primers used to amplify the DNA at the Park14 locus (see Fig. 5 and Table 3 of Slankster et…arrow_forwardFrom your previous experiment, you found that this enhancer activates stripe 2 of eve expression. When you sequence this enhancer you find several binding sites for the gap gene, Giant. To test how Giant interacts with eve, you decide to remove all of the Giant binding sites from the eve enhancer. What results do you expect to see with respect to eve expression?arrow_forward
- What experiment could you do to see if the maternal gene, bicoid, is sufficient to form anterior fates?arrow_forwardYou’re curious about the effect that gap genes have on the pair-rule gene, evenskipped (eve), so you isolate and sequence each of the eve enhancers. You’re particularly interested in one of the enhancers, which is just upstream of the eve gene. Describe an experimental technique you would use to find out where this particular eve enhancer is active.arrow_forwardFor short answer questions, write your answers on the line provided. To the right is the mRNA codon table to use as needed throughout the exam. First letter U บบบ U CA UUCPhe UUA UCU Phe UCC UUG Leu CUU UAU. G U UAC TV UGCys UAA Stop UGA Stop A UAG Stop UGG Trp Ser UCA UCG CCU] 0 CUC CUA CCC CAC CAU His CGU CGC Leu Pro CCA CAA Gin CGA Arg CUG CCG CAG CGG AUU ACU AAU T AUC lle A 1 ACC Thr AUA ACA AUG Mot ACG AGG Arg GUU GCU GUC GCC G Val Ala GAC Asp GGU GGC GUA GUG GCA GCG GAA GGA Gly Glu GAGJ GGG AACASH AGU Ser AAA1 AAG Lys GAU AGA CAL CALUCAO CAO G Third letter 1. (+7) Use the table below to answer the questions; use the codon table above to assist you. The promoter sequence of DNA is on the LEFT. You do not need to fill in the entire table. Assume we are in the middle of a gene sequence (no need to find a start codon). DNA 1 DNA 2 mRNA tRNA Polypeptide C Val G C. T A C a. On which strand of DNA is the template strand (DNA 1 or 2)?_ b. On which side of the mRNA is the 5' end (left or…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license