
The compression ratio of an Otto cycle as shown in Figure 21.12 is VA/VB = 8.00. At the beginning A of the compression process, 500 cm3 of gas is at 100 kPa and 20.0°C. At the beginning of the adiabatic expansion, the temperature is TC = 750°C. Model the working fluid as an ideal gas with γ = 1.40. (a) Fill in this table to follow the states of the gas:

(b) Fill in this table to follow the processes:
(c) Identify the energy input |Qh|, (d) the energy exhaust |Qc|, and (e) the net output work Weng. (f) Calculate the efficiency. (g) Find the number of crankshaft revolutions per minute required for a one-cylinder engine to have an output power of 1.00 kW = 1.34 hp. Note: The
(a)
The states of the gas during the Otto cycle.
Answer to Problem 47CP
The complete table is shown below.
| State | |||
| A | 293 | 100 | 500 |
| B | 673 | 62.5 | |
| C | 1023 | 62.5 | |
| D | 445 | 152 | 500 |
Explanation of Solution
The compression ratio of an Otto cycle is
In Otto cycle, the process
Write the expression to calculate the quantity of the gas.
Here,
Substitute
In process
Write the expression to calculate the pressure at point B.
Here,
Substitute
Write the expression for the compression ratio
Substitute
Write the expression to calculate the temperature at point B.
Substitute
At state C:
Here,
Write the expression to calculate the pressure at point C.
Here,
Substitute
State D:
Here,
Therefore, the compression ratio
Write the expression to calculate the pressure at point D.
Here,
Substitute
Write the expression to calculate the temperature at point D.
Substitute
From the above explanation, the complete table is given below.
| State | |||
| A | 293 | 100 | 500 |
| B | 673 | 62.5 | |
| C | 1023 | 62.5 | |
| D | 445 | 152 | 500 |
Conclusion:
Therefore, the complete table is given below.
| State | |||
| A | 293 | 100 | 500 |
| B | 673 | 62.5 | |
| C | 1023 | 62.5 | |
| D | 445 | 152 | 500 |
(b)
The heat transferred, work done and the change in internal energy during the different process in the Otto cycle.
Answer to Problem 47CP
The complete table is shown below.
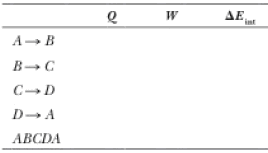
| Process | Q | ||
| 0 | -162 | 162 | |
| 149 | 0 | 149 | |
| 0 | 246 | -246 | |
| -65 | 0 | -65 | |
| 84.3 | 84.3 | 0 |
Explanation of Solution
The process
Let
Write the expression for change in internal energy in A to B process.
Substitute
Write the expression of first law of thermodynamics.
Substitute
Write the expression to calculate the energy in B to C process.
Substitute
Write the expression of first law of thermodynamics.
Substitute
Write the expression to calculate the energy in C to D process.
Substitute
Write the expression of first law of thermodynamics.
Substitute
Write the expression to calculate the energy in D to A process.
Substitute
Write the expression of first law of thermodynamics.
Substitute
Add all the work done found above to find the net work done
Add the heat energy transferred in the four process given above to find the net heat energy
The change in internal energy during a cyclic process is zero.
Thus,
From the above explanation, the complete table is given below.
| Process | Q | ||
| 0 | -162 | 162 | |
| 149 | 0 | 149 | |
| 0 | 246 | -246 | |
| -65 | 0 | -65 | |
| 84.3 | 84.3 | 0 |
Conclusion:
Therefore, the complete table
| Process | Q | ||
| 0 | -162 | 162 | |
| 149 | 0 | 149 | |
| 0 | 246 | -246 | |
| -65 | 0 | -65 | |
| 84.3 | 84.3 | 0 |
(c)
The heat input during
Answer to Problem 47CP
The heat input during
Explanation of Solution
From part (b), the heat input during
Thus, the heat input during
Conclusion:
Therefore, the heat input during
(d)
The heat exhaust during
Answer to Problem 47CP
The heat exhaust during
Explanation of Solution
From part (b)
The heat exhaust during
Thus, the heat exhaust during
Conclusion:
Therefore, the heat exhaust during
(e)
The net work output.
Answer to Problem 47CP
The net work output is
Explanation of Solution
From part (b)
The net work output is
Thus, the net work output is
Conclusion:
Therefore, the net work output is
(f)
The thermal efficiency.
Answer to Problem 47CP
The thermal efficiency is
Explanation of Solution
Write the expression to calculate the thermal efficiency.
Conclusion:
Substitute
Therefore, the thermal efficiency is
(g)
The number of crankshaft revolution per minute.
Answer to Problem 47CP
The number of crankshaft revolution per minute is
Explanation of Solution
Write the expression to calculate the output power.
Here,
Substitute
Thus, the number of crankshaft revolution per minute is
Want to see more full solutions like this?
Chapter 21 Solutions
PHYSICS FOR SCI. & ENGR(LL W/WEBASSIGN)
- 4a Which of the following values COULD NOT be a magnitude? Choose all that apply. 626 0 -0.806 8.63 -48.5 72 131 156 4b Px = -1248 & Py = 261. Determine P.P = Qx = -1540 & Qy = 375. Determine Q.Q = 4c. T = 1105 & Ty = 425. Determine the two possible values for Tx. 4d. Uy = -38. Which of the following COULD NOT be the value of U? Choose all that apply. 10 70 72 31 47 0 75 38 4e. R has a magnitude of 165. Which of the following COULD be Rx? Choose all that apply. 165 -171 155 0 -156 -165 172 -130arrow_forward9.arrow_forward10.arrow_forward
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





