
(a)
To draw a PV diagram for the gas.
(a)
Answer to Problem 42PQ
The PV diagram for the gas is given below.
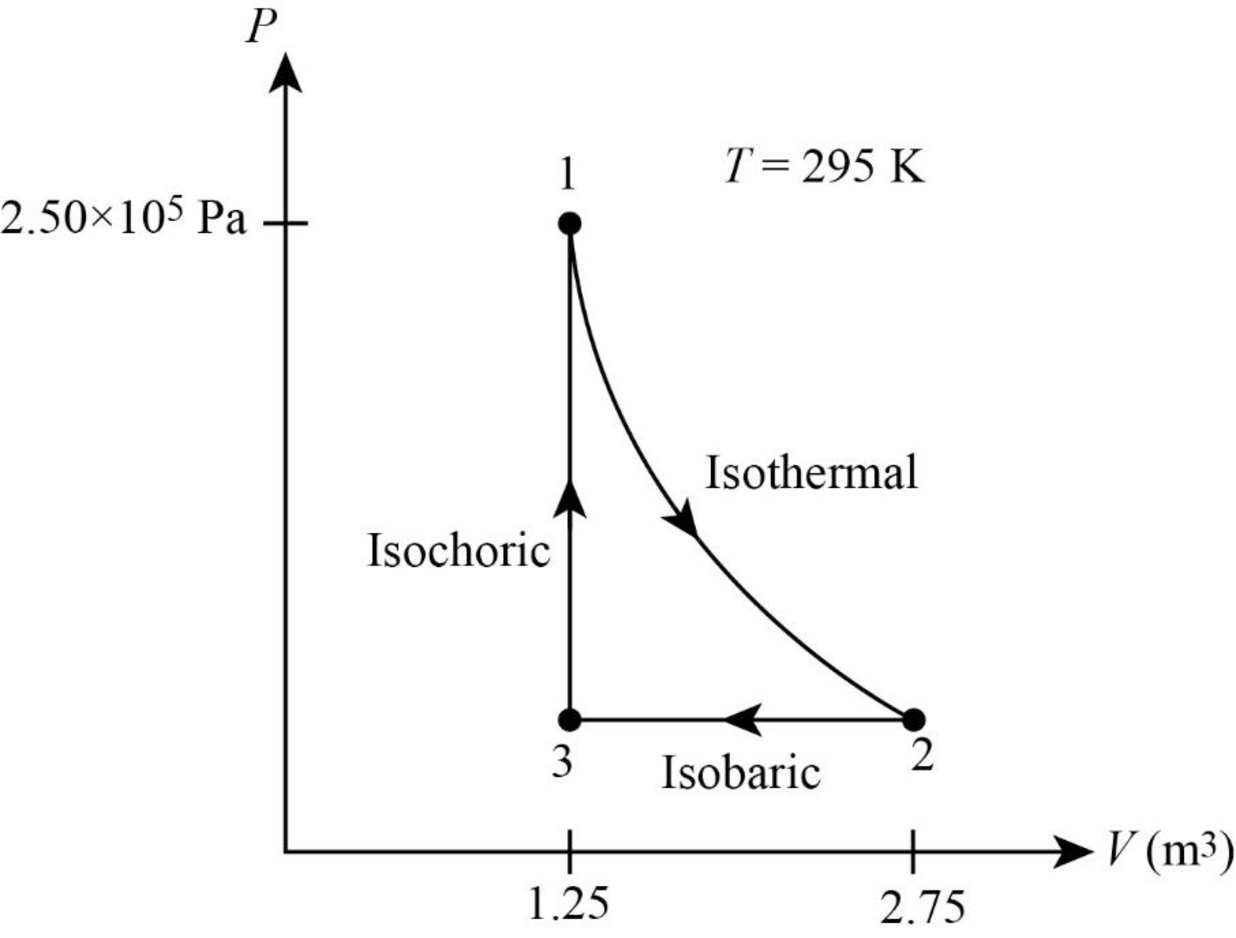
Explanation of Solution
It is given that pressure of the gas is
The following figure gives the PV diagram for the gas.
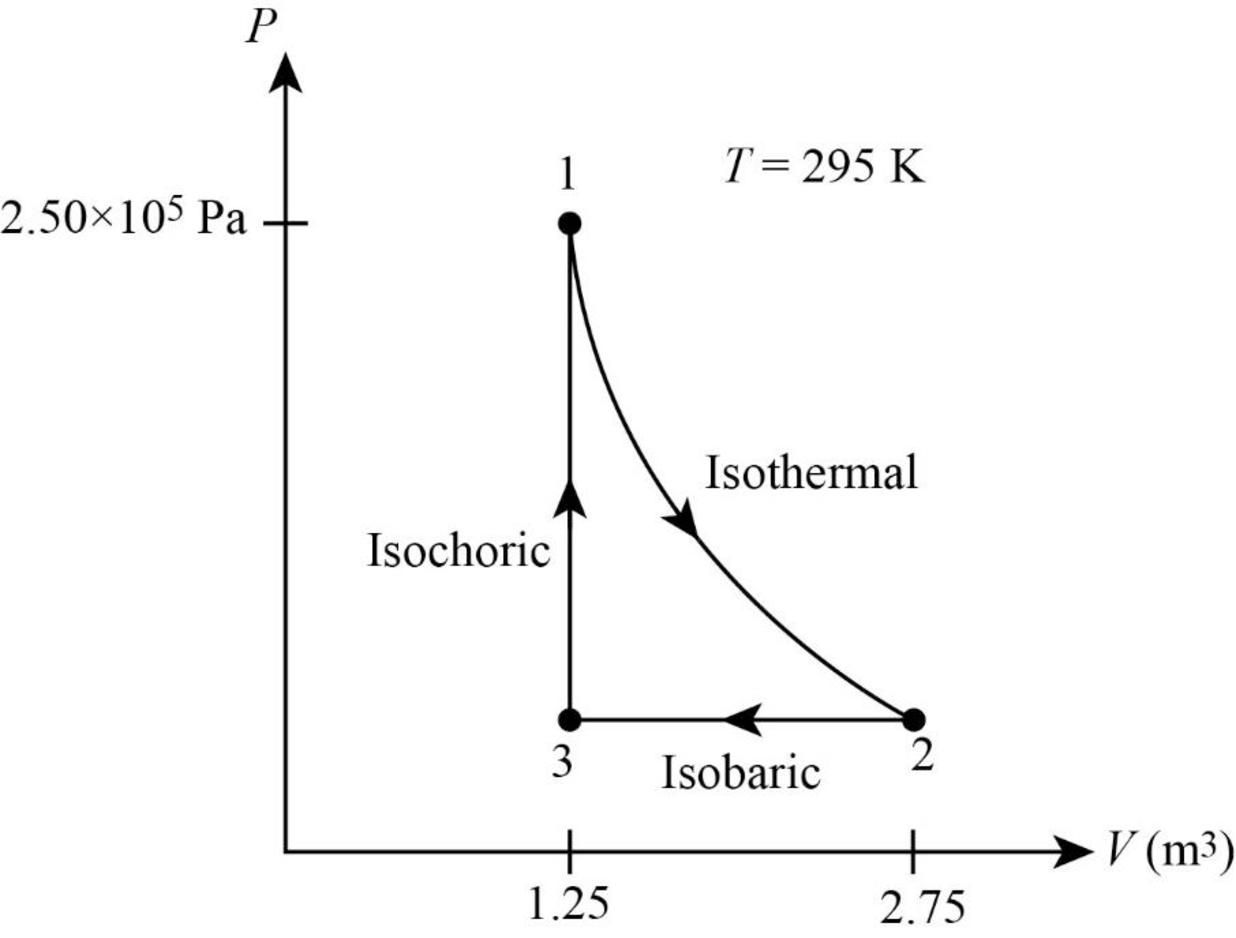
In above figure, point 1 represents the initial state. The gas follows isothermal expansion from
Conclusion:
Thus, the PV diagram for the gas is given below.
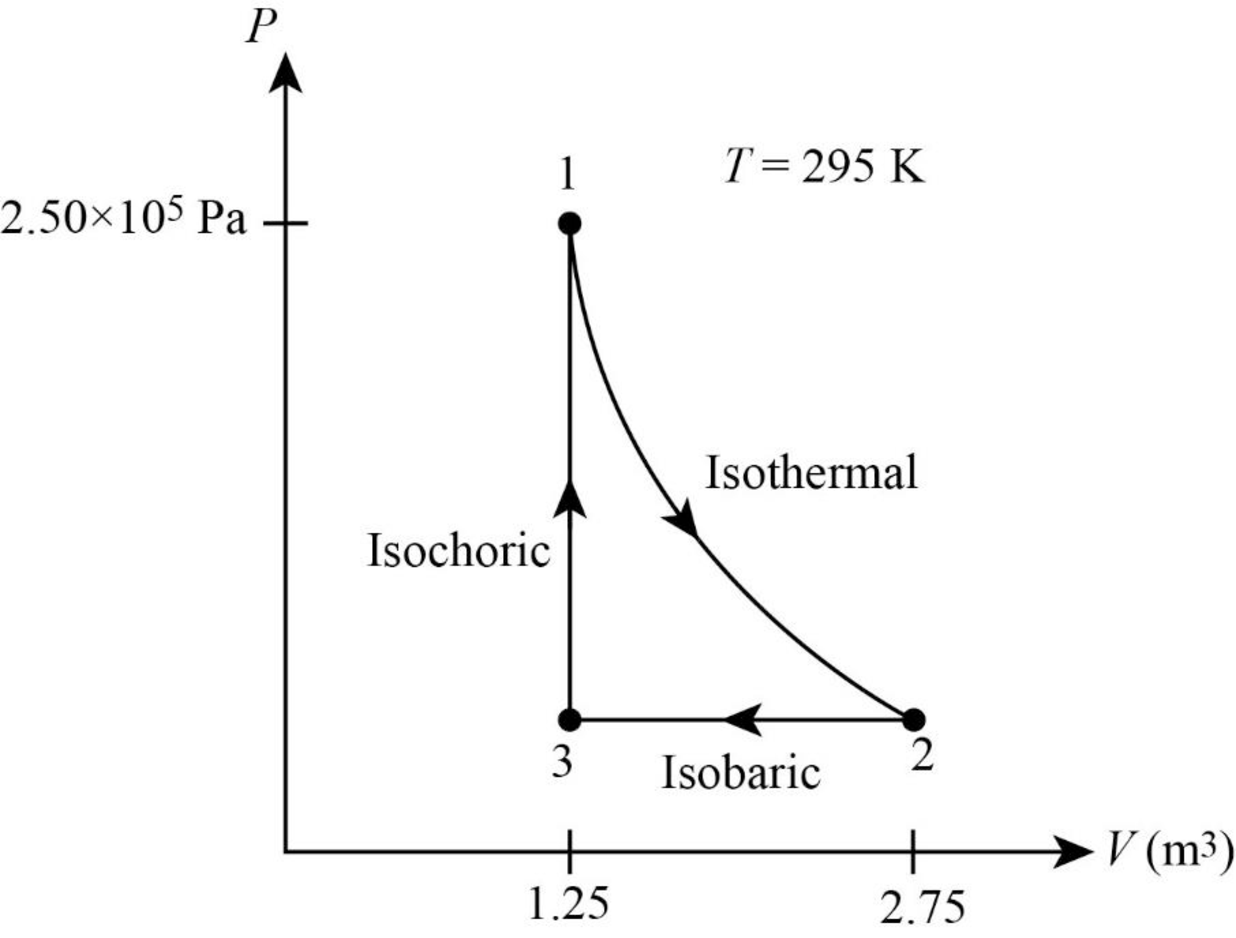
(b)
The change in thermal energy.
(b)
Answer to Problem 42PQ
The change in thermal energy of the gas is zero.
Explanation of Solution
From figure its clear that the gas undergoes a
In this problem it is given that the system return’s to its original state. Thus following a cyclic process, total change in thermal energy of the gas is zero.
Conclusion:
Therefore, the change in thermal energy of the gas is zero.
(c)
The work done by environment on the gas.
(c)
Answer to Problem 42PQ
The work done by environment on the gas is
Explanation of Solution
The net work done by the gas is the area inside the curve. In isothermal process volume of the system is raised from
Write the expression for the work done during isothermal expansion.
Here,
Write ideal gas equation.
Here,
Use above equation for the gas at initial state (state 1 in figure).
Here,
Substitute
For isothermal process,
Apply above equation for the isothermal process of the gas.
Here,
Rearrange above equation to get
In figure1, the straight line represents isobaric process where pressure is constant. During this process the system is compressed to original volume. Therefore, work is done on the system.
Write the expression for the work done in isobaric compression.
Here,
The negative sign indicates that work is done on the gas.
During isobaric process, the gas is at pressure
Use above equation to write work done by gas during isobaric compression shown in figure1.
Here,
In an isochoric process total work done is zero.
Write the expression for the total work done by the gas.
Here,
Conclusion:
Substitute
Substitute
Substitute
Substitute
Therefore, the work done by environment on the gas is
(d)
The heat that flows into the gas.
(d)
Answer to Problem 42PQ
The heat that flows into the gas is
Explanation of Solution
Write the first law of
Here,
Since the process is cyclic,
Substitute
Conclusion:
Substitute
Therefore, the heat that flows into the gas is
Want to see more full solutions like this?
Chapter 21 Solutions
Physics for Scientists and Engineers: Foundations and Connections
- 12. If all three collisions in the figure below are totally inelastic, which will cause more damage? (think about which collision has a larger amount of kinetic energy dissipated/lost to the environment? I m II III A. I B. II C. III m m v brick wall ע ע 0.5v 2v 0.5m D. I and II E. II and III F. I and III G. I, II and III (all of them) 2marrow_forwardCan you solve this 2 question teach me step by step and draw for mearrow_forwardFrom this question and answer can you explain how get (0,0,5) and (5,0,,0) and can you teach me how to solve thisarrow_forward
- Can you solve this 2 question and teach me using ( engineer method formula)arrow_forward11. If all three collisions in the figure below are totally inelastic, which brings the car of mass (m) on the left to a halt? I m II III m m ע ע ע brick wall 0.5v 2m 2v 0.5m A. I B. II C. III D. I and II E. II and III F. I and III G. I, II and III (all of them)arrow_forwardHow can you tell which vowel is being produced here ( “ee,” “ah,” or “oo”)? Also, how would you be able to tell for the other vowels?arrow_forward
- You want to fabricate a soft microfluidic chip like the one below. How would you go about fabricating this chip knowing that you are targeting a channel with a square cross-sectional profile of 200 μm by 200 μm. What materials and steps would you use and why? Disregard the process to form the inlet and outlet. Square Cross Sectionarrow_forward1. What are the key steps involved in the fabrication of a semiconductor device. 2. You are hired by a chip manufacturing company, and you are asked to prepare a silicon wafer with the pattern below. Describe the process you would use. High Aspect Ratio Trenches Undoped Si Wafer P-doped Si 3. You would like to deposit material within a high aspect ratio trench. What approach would you use and why? 4. A person is setting up a small clean room space to carry out an outreach activity to educate high school students about patterning using photolithography. They obtained a positive photoresist, a used spin coater, a high energy light lamp for exposure and ordered a plastic transparency mask with a pattern on it to reduce cost. Upon trying this set up multiple times they find that the full resist gets developed, and they are unable to transfer the pattern onto the resist. Help them troubleshoot and find out why pattern of transfer has not been successful. 5. You are given a composite…arrow_forwardTwo complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find r and θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forward
- An electromagnetic wave is traveling through vacuum in the positive x direction. Its electric field vector is given by E=E0sin(kx−ωt)j^,where j^ is the unit vector in the y direction. If B0 is the amplitude of the magnetic field vector, find the complete expression for the magnetic field vector B→ of the wave. What is the Poynting vector S(x,t), that is, the power per unit area associated with the electromagnetic wave described in the problem introduction? Give your answer in terms of some or all of the variables E0, B0, k, x, ω, t, and μ0. Specify the direction of the Poynting vector using the unit vectors i^, j^, and k^ as appropriate. Please explain all stepsarrow_forwardAnother worker is performing a task with an RWL of only 9 kg and is lifting 18 kg, giving him an LI of 2.0 (high risk). Questions:What is the primary issue according to NIOSH?Name two factors of the RWL that could be improved to reduce risk.If the horizontal distance is reduced from 50 cm to 30 cm, how does the HM change and what effect would it have?arrow_forwardTwo complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find r and θ for z1z2∗. Find r and θ for z1/z2∗? Find r and θ for (z1−z2)∗/z1+z2∗. Find r and θ for (z1−z2)∗/z1z2∗ Please explain all steps, Thank youarrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





