
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
12th Edition
ISBN: 9781119664635
Author: Solomons
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 27P
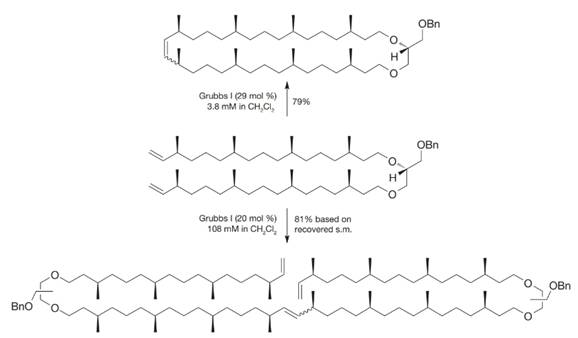
When the following molecule was exposed to the Grubbs I initiator at different concentrations, different products resulted. How can you account for these different outcomes?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
QUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression'
*The images of the data showing 'coefficients for the standard curve' have been provided
Using the Nernst equation to calculate nonstandard cell voltage
Try Again
Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations.
A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
2+
2+
Sn²+ Ba(s)
(aq) + Ba (s) Sn (s) + Ba²+ (aq)
→>>
Suppose the cell is prepared with 6.10 M Sn
2+
2+
in one half-cell and 6.62 M Ba
in the other.
Calculate the cell voltage under these conditions. Round your answer to 3 significant digits.
1.71 V
☐ x10
☑
5
0/5
?
00.
18
Ar
Question: Find both the b (gradient) and a (y-intercept) value from the list of data below:
(x1 -x̄)
370.5
(y1 - ȳ)
5.240
(x2 - x̄)
142.5
(y2 - ȳ)
2.004
(x3 - x̄)
28.5
(y3 - ȳ)
0.390
(x4 - x̄)
-85.5
(y4 - ȳ)
-1.231
(x5 - x̄)
-199.5
(y5 - ȳ)
-2.829
(x6 - x̄)
-256.5
(y6 - ȳ)
-3.575
Chapter 21 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Ch. 21 - PRACTICE PROBLEM 21.1
For each of the following...Ch. 21 - Prob. 2PPCh. 21 - PRACTICE PROBLEM 21.3 What product would you...Ch. 21 - Prob. 4PPCh. 21 - PRACTICE PROBLEM 21.5 What is the product of the...Ch. 21 - Prob. 6PPCh. 21 - Prob. 7PPCh. 21 - Prob. 8PPCh. 21 - Prob. 9PPCh. 21 - Prob. 10PP
Ch. 21 - Prob. 11PPCh. 21 - Practice Problem 21.12 What products would form...Ch. 21 - Prob. 13PCh. 21 - Prob. 14PCh. 21 - How would you synthesize each of the following...Ch. 21 - Prob. 16PCh. 21 - Predict the product(s) for each of the following...Ch. 21 - Prob. 18PCh. 21 - Prob. 19PCh. 21 - Prob. 20PCh. 21 - Prob. 21PCh. 21 - 21.22 Write a mechanism that can account for the...Ch. 21 - Prob. 23PCh. 21 - Prob. 24PCh. 21 - Prob. 25PCh. 21 - 21.26 In 1985, T. Katz (Columbia University)...Ch. 21 - When the following molecule was exposed to the...Ch. 21 - During the course of the following Stille...Ch. 21 - 1. In “The Chemistry of... Complex Cross...Ch. 21 - Prob. 2LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
WRITE ABOUT A THEME: ENERGY In a short essay (about 100150 words), discuss how prokaryotes and other members of...
Campbell Biology (11th Edition)
2.81 In which of the fo1losing pairs do both numbers contain the same number of significant figures? (2.2)
a....
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
The following results were obtained from a broth dilution test for microbial susceptibility. Antibiotic Concent...
Microbiology: An Introduction
PRACTICE 1.3 The melting point of table salt is 1474oF. What temperature is this on the Celsius and Kelvin scal...
Chemistry (7th Edition)
Given the end results of the two types of division, why is it necessary for homologs to pair during meiosis and...
Concepts of Genetics (12th Edition)
Did all the organisms living in or on the environments sampled grow on your nutrient agar? Briefly explain.
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forward
- The following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forwardBriefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forward
- Explain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward
- 7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardGive reason(s) for six from the followings [using equations if possible] a. Addition of sodium carbonate to sulfanilic acid in the Methyl Orange preparation. b. What happened if the diazotization reaction gets warmed up by mistake. c. Addition of sodium nitrite in acidified solution in MO preparation through the diazotization d. Using sodium dithionite dihydrate in the second step for Luminol preparation. e. In nitroaniline preparation, addition of the acid mixture (nitric acid and sulfuric acid) to the product of step I. f. What is the main reason of the acylation step in nitroaniline preparation g. Heating under reflux. h. Fusion of an organic compound with sodium. HAND WRITTEN PLEASEarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY