
GENERAL, ORGANIC, BIOCHEM (LL W/ ACCESS)
10th Edition
ISBN: 9781260885958
Author: Denniston
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 21, Problem 21.54QP
Interpretation Introduction
Interpretation:
The intermediate that is formed in each of the following reactions has to be drawn.
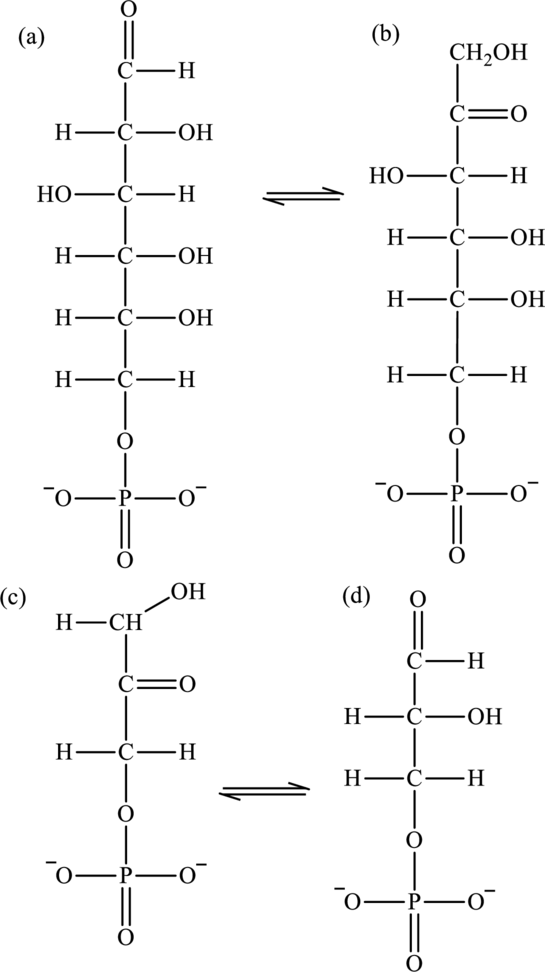
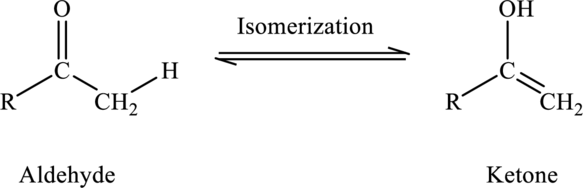
Concept Introduction:
Isomerization reaction is a reaction that converts one isomer into another. For example, isomerization of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Assign these C-NMR and H-NMR Spectrum
Predict the product of this organic reaction:
IZ
+
HO
i
P+H₂O
Specifically, in the drawing area below draw the skeletal ("line") structure of P.
If there is no reasonable possibility for P, check the No answer box under the drawing area.
No Answer
Click and drag to start drawing a
structure.
☐ :
Predict the products of this organic reaction:
0
O
-----
A
+ KOH ?
CH3-CH2-C-O-CH2-C-CH3
Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them
in any arrangement you like, so long as they aren't touching.)
If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area.
No reaction
Click anywhere to draw the first
atom of your structure.
X
⑤
è
Chapter 21 Solutions
GENERAL, ORGANIC, BIOCHEM (LL W/ ACCESS)
Ch. 21.1 - Prob. 21.1QCh. 21.1 - Prob. 21.2QCh. 21.2 - Prob. 21.3QCh. 21.2 - Prob. 21.4QCh. 21.3 - Prob. 21.5QCh. 21.3 - Prob. 21.6QCh. 21.3 - Prob. 21.7QCh. 21.3 - Prob. 21.8QCh. 21.4 - Prob. 21.9QCh. 21.4 - Prob. 21.10Q
Ch. 21.6 - Prob. 21.11QCh. 21.6 - Prob. 21.12QCh. 21.7 - Prob. 21.13QCh. 21.7 - Prob. 21.14QCh. 21.7 - Prob. 21.15QCh. 21.7 - Prob. 21.16QCh. 21.7 - Prob. 21.17QCh. 21.7 - Prob. 21.18QCh. 21 - Prob. 21.19QPCh. 21 - Prob. 21.20QPCh. 21 - Prob. 21.21QPCh. 21 - Prob. 21.22QPCh. 21 - Prob. 21.23QPCh. 21 - Prob. 21.24QPCh. 21 - Prob. 21.25QPCh. 21 - Prob. 21.26QPCh. 21 - Prob. 21.27QPCh. 21 - Prob. 21.28QPCh. 21 - Write a balanced equation showing the hydrolysis...Ch. 21 - Prob. 21.30QPCh. 21 - Prob. 21.31QPCh. 21 - Prob. 21.32QPCh. 21 - Prob. 21.33QPCh. 21 - Prob. 21.34QPCh. 21 - Prob. 21.35QPCh. 21 - Prob. 21.36QPCh. 21 - Prob. 21.37QPCh. 21 - Prob. 21.38QPCh. 21 - Prob. 21.39QPCh. 21 - Prob. 21.40QPCh. 21 - Prob. 21.41QPCh. 21 - Prob. 21.42QPCh. 21 - Prob. 21.43QPCh. 21 - Prob. 21.44QPCh. 21 - Prob. 21.47QPCh. 21 - Prob. 21.48QPCh. 21 - Prob. 21.49QPCh. 21 - Prob. 21.50QPCh. 21 - Prob. 21.51QPCh. 21 - Prob. 21.52QPCh. 21 - Prob. 21.53QPCh. 21 - Prob. 21.54QPCh. 21 - Prob. 21.55QPCh. 21 - Prob. 21.56QPCh. 21 - Prob. 21.57QPCh. 21 - Prob. 21.58QPCh. 21 - Prob. 21.59QPCh. 21 - Prob. 21.60QPCh. 21 - Prob. 21.61QPCh. 21 - Prob. 21.62QPCh. 21 - Prob. 21.63QPCh. 21 - Prob. 21.64QPCh. 21 - Prob. 21.65QPCh. 21 - Prob. 21.66QPCh. 21 - Prob. 21.67QPCh. 21 - Prob. 21.68QPCh. 21 - Prob. 21.69QPCh. 21 - Prob. 21.70QPCh. 21 - Prob. 21.71QPCh. 21 - Prob. 21.72QPCh. 21 - Prob. 21.73QPCh. 21 - Prob. 21.74QPCh. 21 - Prob. 21.75QPCh. 21 - Prob. 21.76QPCh. 21 - Prob. 21.77QPCh. 21 - Prob. 21.78QPCh. 21 - Prob. 21.79QPCh. 21 - Prob. 21.80QPCh. 21 - Prob. 21.81QPCh. 21 - Prob. 21.82QPCh. 21 - Prob. 21.83QPCh. 21 - Prob. 21.84QPCh. 21 - Prob. 21.85QPCh. 21 - Prob. 21.86QPCh. 21 - Prob. 21.87QPCh. 21 - Prob. 21.88QPCh. 21 - Prob. 21.89QPCh. 21 - Prob. 21.90QPCh. 21 - Prob. 21.91QPCh. 21 - What enzyme in glycogen metabolism is stimulated...Ch. 21 - Prob. 21.93QPCh. 21 - Prob. 21.94QPCh. 21 - Prob. 21.95QPCh. 21 - Describe the function of the debranching enzyme in...Ch. 21 - Write a balanced equation for the reaction...Ch. 21 - Prob. 21.98QPCh. 21 - Prob. 21.99QPCh. 21 - Prob. 21.100QPCh. 21 - Prob. 6MCPCh. 21 - Prob. 7MCPCh. 21 - Prob. 8MCPCh. 21 - Prob. 9MCPCh. 21 - Prob. 10MCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
- You are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forwardPredict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY