
Concept explainers
(a)
Interpretation:
From the following hydrocarbons, alkane is to be identified.
Concept introduction:
Hydrocarbons contain carbon and hydrogen atoms.
Answer to Problem 21.3TC
Among the given hydrocarbons
Explanation of Solution
Alkanes are the saturated hydrocarbons in which each carbon atom is bonded to four other atoms via single bond only. The alkanes general formula of is
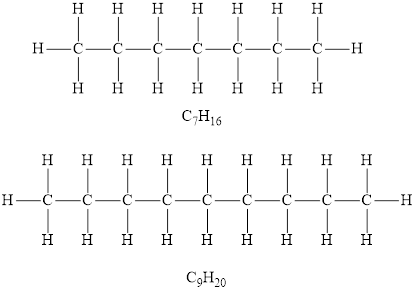
According to the general formula of alkanes, for 7 carbon atoms there must be 16 hydrogen atoms and for 9 carbon atoms, there must be 20 hydrogen atoms. Hydrocarbons
According to the general formula of alkanes, for 5 carbon atoms there must be 12 hydrogen atoms and for 11 carbon atoms, there must be 24 hydrogen atoms. Hydrocarbons
Among the given hydrocarbons
(b)
Interpretation:
The formula of the alkyl group derived from pentane is to be identified.
Concept introduction:
An alkyl group is derived from the respective alkane. Alkyl group is an alkane with one hydrogen atom less than the respective alkane. The alkyl group is named after alkane counterpart containing the same number of carbon atoms by replacing the “ane” suffix to “yl”.
Answer to Problem 21.3TC
The formula of the alkyl group obtained from pentane is
Explanation of Solution
The molecular formula of pentane is
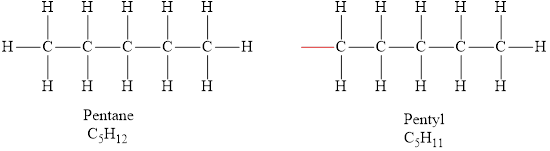
The formula of the alkyl group derived from pentane is
(c)
Interpretation:
The structural diagram of
Concept introduction:
Lewis diagram is the representation of the molecules or ions. It shows the bonding between the atoms and also shows the unshared pairs of electrons on an atom if any. The structural formula or structural diagram is nothing but Lewis structure without unshared pairs of electrons.
In the IUPAC name of an organic compound represents the number of the substituent and the numbers before the Greek prefix represent the position of the substituents. The suffix for an alkane is ‘ane. The number of carbon atoms in the parent hydrocarbon chain is represented by the root name. For example: ‘prop’ represent 3 carbon atoms present in the parent hydrocarbon chain.
Answer to Problem 21.3TC
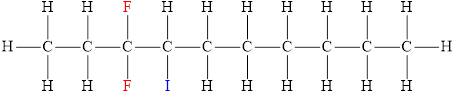
The structural diagram of
Explanation of Solution
The IUPAC name of the hydrocarbon is
The structura diagram is,
The structural diagram of

(d)
Interpretation:
The structural diagram of
Concept introduction:
Lewis diagram is the representation of the molecule or ion. It shows the bonding between the atoms and also shows the unshared pairs of electrons on an atom if any. The structural formula or structural diagram is nothing but Lewis structure without unshared pairs of electrons.
In the IUPAC name of an organic compound, the Greek prefix represents the number of the substituent and the numbers before the Greek prefix represent the position of the substituents. The suffix for an alkane is ‘ane and the prefix ‘cyclo’ is used for cycloalkanes. The number of carbon atoms in the parent hydrocarbon chain is represented by the root name. For example: ‘prop’ represent 3 carbon atoms present in the parent hydrocarbon chain.
Answer to Problem 21.3TC
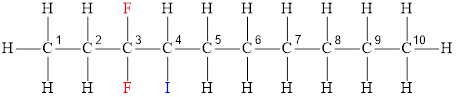
The structural diagram of
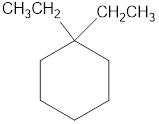
Explanation of Solution
The IUPAC name of the hydrocarbon is
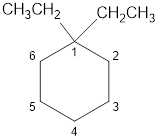
The structural diagram of

Want to see more full solutions like this?
Chapter 21 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + OWLv2, 1 term (6 months) Printed Access Card
- By malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forwardEFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
- If we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forwardQUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forward
- You need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forwardQUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





