
a)
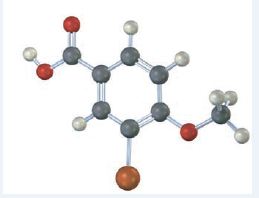
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
The IUPAC name is 3-bromo-4-methoxybenzoic acid.
Explanation of Solution
The compound represented by the model is
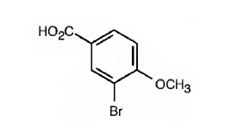
It is a benzoic acid derivative with a bromine atom on C3 and a methoxyl group on C4.
The IUPAC name of the compound represented by the model shown is 3-bromo-4-methoxybenzoic acid.
b)
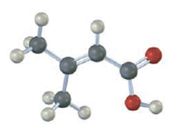
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
The IUPAC name of the compound represented by the model shown is 3-methyl-2-butenoic acid.
Explanation of Solution
The compound represented by the model is
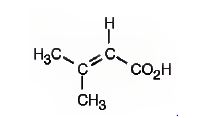
The compound is an unsaturated acid having a four carbon straight chain with a double bond between C2 and C3.
The IUPAC name of the compound represented by the model shown is 3-methyl-2-butenoic acid.
c)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
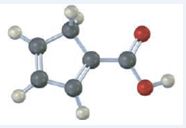
The IUPAC name of the compound represented by the model shown is cyclopenta-1,3-dienecarboxylic acid.
Explanation of Solution
The compound represented by the model is
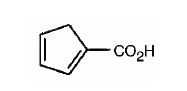
The compound has a cyclopentadiene ring to which a carboxyl group is attached. The double bonds in the diene are in between C1 & C2 and C3 & C4.
The IUPAC name of the compound represented by the model shown is cyclopenta-1,3-dienecarboxylic acid.
d)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
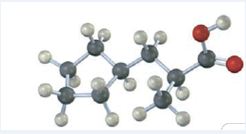
The IUPAC name of the compound represented by the model shown is (S)-3-cyclopentyl-2-methylpropanoic acid.
Explanation of Solution
The compound represented by the model is
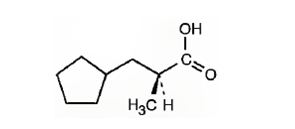
The compound is a carboxylic acid with a three carbon straight chin and has a methyl group attached to C2 and cyclopentyl group attached to C3. The compound is optically active as C2 is chiral. The groups of first highest, second highest and third highest groups when viewed away from the group of last priority are arranged in the anti-clockwise direction. Hence the compound has S stereochemistry.
The IUPAC name of the compound represented by the model shown is (S)-3-cyclopentyl-2-methylpropanoic acid.
Want to see more full solutions like this?
Chapter 20 Solutions
EBK ORGANIC CHEMISTRY
- Can I get helpp drawing my arrowsarrow_forwardWhich of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forward
- I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forwardWe discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forward

 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

