
Physics for Scientists and Engineers with Modern Physics
4th Edition
ISBN: 9780136139225
Author: Douglas C. Giancoli
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 6P
(II) Figure 20–17 is a PV diagram for a reversible
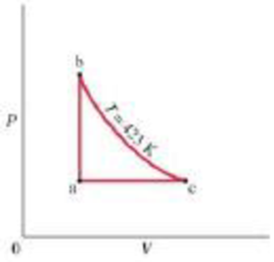
FIGURE 20–17
Problem 6.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Find the amplitude, wavelength, period, and the speed of the wave.
A long solenoid of length 6.70 × 10-2 m and cross-sectional area 5.0 × 10-5 m² contains
6500 turns per meter of length. Determine the emf induced in the solenoid when the
current in the solenoid changes from 0 to 1.5 A during the time interval from 0 to 0.20 s.
Number
Units
A coat hanger of mass m = 0.255 kg oscillates on a peg as a physical pendulum as shown in the figure below. The distance from the pivot to the center of mass of the coat hanger is d = 18.0 cm and the period of the motion is T = 1.37 s. Find the moment of inertia of the coat hanger about the pivot.
Chapter 20 Solutions
Physics for Scientists and Engineers with Modern Physics
Ch. 20.2 - An adiabatic process is defined as one in which no...Ch. 20.3 - A motor is running with an intake temperature TH =...Ch. 20.6 - A 1.00.kg piece of ice at 0C melts very slowly to...Ch. 20.9 - Prob. 1EECh. 20 - Prob. 1QCh. 20 - Can you warm a kitchen in winter by leaving the...Ch. 20 - Would a definition of heat engine efficiency as e...Ch. 20 - What plays the role of high-temperature and...Ch. 20 - Which will give the greater improvement in the...Ch. 20 - The oceans contain a tremendous amount of thermal...
Ch. 20 - Discuss the factors that keep real engines from...Ch. 20 - Prob. 8QCh. 20 - Describe a process in nature that is nearly...Ch. 20 - (a) Describe how heat could be added to a system...Ch. 20 - Suppose a gas expands to twice its original volume...Ch. 20 - Give three examples, other than those mentioned in...Ch. 20 - Which do you think has the greater entropy, 1 kg...Ch. 20 - (a) What happens if you remove the lid of a bottle...Ch. 20 - Prob. 15QCh. 20 - Prob. 16QCh. 20 - Prob. 17QCh. 20 - The first law of thermodynamics is sometimes...Ch. 20 - Powdered milk is very slowly (quasistatically)...Ch. 20 - Two identical systems are taken from state a to...Ch. 20 - It can he said that the total change in entropy...Ch. 20 - Use arguments, other than the principle of entropy...Ch. 20 - (I) A heat engine exhausts 7800 J of heat while...Ch. 20 - (I) A certain power plant puts out 580 MW of...Ch. 20 - (II) A typical compact car experiences a total...Ch. 20 - (II) A four-cylinder gasoline engine has an...Ch. 20 - (II) The burning of gasoline in a car releases...Ch. 20 - (II) Figure 2017 is a PV diagram for a reversible...Ch. 20 - (III) The operation of a diesel engine can be...Ch. 20 - (I) What is the maximum efficiency of a heat...Ch. 20 - (I) It is not necessary that a heat engines hot...Ch. 20 - (II) A heal engine exhausts its heat at 340C and...Ch. 20 - (II) (a) Show that the work done by a Carnot...Ch. 20 - (II) A Carnot engines operating temperatures are...Ch. 20 - (II) A nuclear power plant operates at 65% of its...Ch. 20 - (II) A Carnot engine performs work at the rate of...Ch. 20 - (II) Assume that a 65 kg hiker needs 4.0 103 kcal...Ch. 20 - (II) A particular car does work at the rate of...Ch. 20 - (II) A heat engine utilizes a heat source at 580C...Ch. 20 - (II) The working substance of a certain Carnot...Ch. 20 - (III) A Carnot cycle, shown in Fig. 20-7, has the...Ch. 20 - (III) One mole of monatomic gas undergoes a Carnot...Ch. 20 - (III) In an engine that approximates the Otto...Ch. 20 - (I) If an ideal refrigerator keeps its contents at...Ch. 20 - (I) The low temperature of a freezer cooling coil...Ch. 20 - (II) An ideal (Carnot) engine has an efficiency of...Ch. 20 - (II) An ideal heal pump is used to maintain the...Ch. 20 - (II) A restaurant refrigerator has a coefficient...Ch. 20 - (II) A heat pump is used to keep a house warm at...Ch. 20 - (II) (a) Given that the coefficient of performance...Ch. 20 - (II) A Carnot refrigerator (reverse of a Carnot...Ch. 20 - (II) A central heat pump updating as an air...Ch. 20 - (II) What volume of water at 0C can a freezer make...Ch. 20 - (I) What is the change in entropy of 250g of steam...Ch. 20 - (I) A 7.5-kg box having an initial speed of 4.0m/s...Ch. 20 - (I) What is the change in entropy of 1.00 m3 of...Ch. 20 - (II) If 1.00m3 of water at 0C is frozen and cooled...Ch. 20 - (II) If 0.45kg f water at 100C is changed by a...Ch. 20 - (II) An aluminum rod conducts 9.50 cal/s from a...Ch. 20 - (II) A 2.8-kg piece of aluminum at 43.0C is placed...Ch. 20 - (II) An ideal gas expands isothermally (T = 410 K)...Ch. 20 - (II) When 2.0 kg of water at 12.0C is mixed with...Ch. 20 - (II) (a) An ice cube of mass m at 0C is placed in...Ch. 20 - (II) The temperature of 2.0mol of an ideal...Ch. 20 - (II) Calculate the change in entropy of 1.00kg of...Ch. 20 - (II) An ideal gas of n moles undergoes the...Ch. 20 - (II) Two samples of an ideal gas are initially at...Ch. 20 - (II) A 150-g insulated aluminum cup at 15C is...Ch. 20 - (II) (a) Why would you expect the total entropy...Ch. 20 - (II) 1.00 mole of nitrogen (N2) gas and 1.00 mole...Ch. 20 - (II) Thermodynamic processes are sometimes...Ch. 20 - (III) The specific heat per mole of potassium at...Ch. 20 - (III) Consider an ideal gas of n moles with molar...Ch. 20 - (III) A general theorem states that the amount of...Ch. 20 - (III) Determine the work available in a 3.5-kg...Ch. 20 - (I) Use Eq. 2014 to determine the entropy of each...Ch. 20 - (II) Suppose that you repeatedly shake six coins...Ch. 20 - (II) Calculate the relative probabilities, when...Ch. 20 - (II) (a) Suppose you have four coins, all with...Ch. 20 - Prob. 58PCh. 20 - (II) Energy may be stored for use during peak...Ch. 20 - (II) Solar cells (Fig. 20-22) can produce about...Ch. 20 - Prob. 61PCh. 20 - It has been suggested that a heat engine could be...Ch. 20 - A heat engine takes a diatomic gas around the...Ch. 20 - A 126.5-g insulated aluminum cup at 18.00C is...Ch. 20 - (a) At a steam power plant, steam engines work in...Ch. 20 - (II) Refrigeration units can be rated in tons. A...Ch. 20 - Prob. 67GPCh. 20 - (a) What is the coefficient of performance of an...Ch. 20 - The operation of a certain heat engine takes an...Ch. 20 - A car engine whose output power is 155 hp operates...Ch. 20 - Suppose a power plant delivers energy at 850 MW...Ch. 20 - 1.00 mole of an ideal monatomic gas at STP first...Ch. 20 - Two 1100-kg cars are traveling 75 km/h in opposite...Ch. 20 - Metabolizing 1.0 kg of fat results in about 3.7 ...Ch. 20 - A cooling unit for a new freezer has an inner...Ch. 20 - Prob. 76GPCh. 20 - The Stirling cycle shown in Fig 20-27, is useful...Ch. 20 - A gas turbine operates under the Brayton cycle,...Ch. 20 - Thermodynamic processes can be represented not...Ch. 20 - An aluminum can, with negligible heat capacity, is...Ch. 20 - Prob. 81GPCh. 20 - A bowl contains a large number of red, orange, and...
Additional Science Textbook Solutions
Find more solutions based on key concepts
What global policy changes and what individual choices can help us sustain the planet that sustains us?
Biology: Life on Earth with Physiology (11th Edition)
WH AT IF? Suppose two plant populations exchange pollen and seeds. In one population, individuals of geno-type...
Campbell Biology (11th Edition)
With what geologic feature are the earthquakes in the mid-Atlantic associated?
Applications and Investigations in Earth Science (9th Edition)
Match each of the following items with all the terms it applies to:
Human Physiology: An Integrated Approach (8th Edition)
What two body structures contain flexible elastic cartilage?
Anatomy & Physiology (6th Edition)
Modified True/False 6. __________ Halophiles inhabit extremely saline habitats, such as the Great Salt Lake.
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Review Conceptual Example 3 and the drawing as an aid in solving this problem. A conducting rod slides down between two frictionless vertical copper tracks at a constant speed of 3.9 m/s perpendicular to a 0.49-T magnetic field. The resistance of th rod and tracks is negligible. The rod maintains electrical contact with the tracks at all times and has a length of 1.4 m. A 1.1-Q resistor is attached between the tops of the tracks. (a) What is the mass of the rod? (b) Find the change in the gravitational potentia energy that occurs in a time of 0.26 s. (c) Find the electrical energy dissipated in the resistor in 0.26 s.arrow_forwardA camera lens used for taking close-up photographs has a focal length of 21.5 mm. The farthest it can be placed from the film is 34.0 mm. (a) What is the closest object (in mm) that can be photographed? 58.5 mm (b) What is the magnification of this closest object? 0.581 × ×arrow_forwardGiven two particles with Q = 4.40-µC charges as shown in the figure below and a particle with charge q = 1.40 ✕ 10−18 C at the origin. (Note: Assume a reference level of potential V = 0 at r = ∞.) Three positively charged particles lie along the x-axis of the x y coordinate plane.Charge q is at the origin.Charge Q is at (0.800 m, 0).Another charge Q is at (−0.800 m, 0).(a)What is the net force (in N) exerted by the two 4.40-µC charges on the charge q? (Enter the magnitude.) N(b)What is the electric field (in N/C) at the origin due to the two 4.40-µC particles? (Enter the magnitude.) N/C(c)What is the electrical potential (in kV) at the origin due to the two 4.40-µC particles? kV(d)What If? What would be the change in electric potential energy (in J) of the system if the charge q were moved a distance d = 0.400 m closer to either of the 4.40-µC particles?arrow_forward
- (a) Where does an object need to be placed relative to a microscope in cm from the objective lens for its 0.500 cm focal length objective to produce a magnification of -25? (Give your answer to at least three decimal places.) 0.42 × cm (b) Where should the 5.00 cm focal length eyepiece be placed in cm behind the objective lens to produce a further fourfold (4.00) magnification? 15 × cmarrow_forwardIn a LASIK vision correction, the power of a patient's eye is increased by 3.10 D. Assuming this produces normal close vision, what was the patient's near point in m before the procedure? (The power for normal close vision is 54.0 D, and the lens-to-retina distance is 2.00 cm.) 0.98 x marrow_forwardDon't use ai to answer I will report you answerarrow_forward
- A shopper standing 2.00 m from a convex security mirror sees his image with a magnification of 0.200. (Explicitly show on paper how you follow the steps in the Problem-Solving Strategy for mirrors found on page 1020. Your instructor may ask you to turn in this work.) (a) Where is his image (in m)? (Use the correct sign.) -0.4 m in front of the mirror ▾ (b) What is the focal length (in m) of the mirror? -0.5 m (c) What is its radius of curvature (in m)? -1.0 marrow_forwardAn amoeba is 0.309 cm away from the 0.304 cm focal length objective lens of a microscope.arrow_forwardTwo resistors of resistances R1 and R2, with R2>R1, are connected to a voltage source with voltage V0. When the resistors are connected in series, the current is Is. When the resistors are connected in parallel, the current Ip from the source is equal to 10Is. Let r be the ratio R1/R2. Find r. I know you have to find the equations for V for both situations and relate them, I'm just struggling to do so. Please explain all steps, thank you.arrow_forward
- Bheem and Ram, jump off either side of a bridge while holding opposite ends of a rope and swing back and forth under the bridge to save a child while avoiding a fire. Looking at the swing of just Bheem, we can approximate him as a simple pendulum with a period of motion of 5.59 s. How long is the pendulum ? When Bheem swings, he goes a full distance, from side to side, of 10.2 m. What is his maximum velocity? What is his maximum acceleration?arrow_forwardThe position of a 0.300 kg object attached to a spring is described by x=0.271 m ⋅ cos(0.512π⋅rad/s ⋅t) (Assume t is in seconds.) Find the amplitude of the motion. Find the spring constant. Find the position of the object at t = 0.324 s. Find the object's velocity at t = 0.324 s.arrow_forwardMin Min is hanging from her spring-arms off the edge of the level. Due to the spring like nature of her arms she is bouncing up and down in simple harmonic motion with a maximum displacement from equilibrium of 0.118 m. The spring constant of Min-Min’s arms is 9560. N/m and she has a mass of 87.5 kg. What is the period at which she oscillates? Find her maximum speed. Find her speed when she is located 5.00 cm from her equilibrium position.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY