
Concept explainers
Compound W
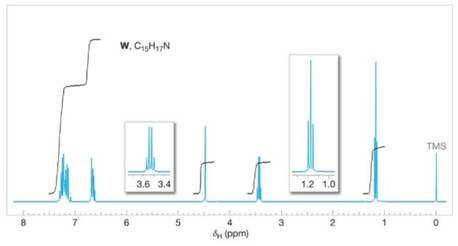
Figure 20.6 The
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Additional Science Textbook Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Organic Chemistry (8th Edition)
Microbiology: An Introduction
Anatomy & Physiology (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- For each of the Followin, moleaks draw all OF The Resonance contributing stuluctures and compare these three molecules in terms of Resonance stabilization 1-C-1 a. b. H A-C+ О 112-1 C. F-C-F Farrow_forwarda. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forwardC. Ν Harrow_forward
- a. H3C. N H3C CH3 HCNarrow_forwardол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


