
Concept explainers
(a)
The current in the metal rod.
(a)
Answer to Problem 3P
The current in the metal rod is
Explanation of Solution
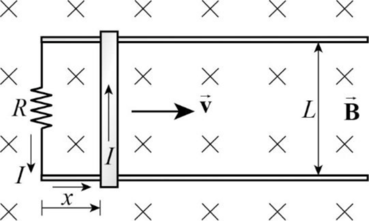
Figure
Write the expression for the induced emf in the metal rod.
Here,
Write the expression for the current in the metal rod.
Here,
Use equation (II) in (I) to solve for
Conclusion:
Substitute
Therefore, the current in the metal rod is
(b)
The energy dissipated in the resistor.
(b)
Answer to Problem 3P
The rate of energy dissipated in the resistor is
Explanation of Solution
Write the expression for the energy dissipated in the resistor.
Here,
Conclusion:
Substitute
Therefore, the energy dissipated in the resistor is
(c)
The magnetic force on the metal rod.
(c)
Answer to Problem 3P
The magnetic force on the metal rod is
Explanation of Solution
Write the expression for the magnetic force.
Here,
Conclusion:
Substitute
Therefore, the magnetic force on the metal rod is
Want to see more full solutions like this?
Chapter 20 Solutions
PHYSICS
- Don't use ai to answer I will report you answerarrow_forwardA shopper standing 2.00 m from a convex security mirror sees his image with a magnification of 0.200. (Explicitly show on paper how you follow the steps in the Problem-Solving Strategy for mirrors found on page 1020. Your instructor may ask you to turn in this work.) (a) Where is his image (in m)? (Use the correct sign.) -0.4 m in front of the mirror ▾ (b) What is the focal length (in m) of the mirror? -0.5 m (c) What is its radius of curvature (in m)? -1.0 marrow_forwardAn amoeba is 0.309 cm away from the 0.304 cm focal length objective lens of a microscope.arrow_forward
- Two resistors of resistances R1 and R2, with R2>R1, are connected to a voltage source with voltage V0. When the resistors are connected in series, the current is Is. When the resistors are connected in parallel, the current Ip from the source is equal to 10Is. Let r be the ratio R1/R2. Find r. I know you have to find the equations for V for both situations and relate them, I'm just struggling to do so. Please explain all steps, thank you.arrow_forwardBheem and Ram, jump off either side of a bridge while holding opposite ends of a rope and swing back and forth under the bridge to save a child while avoiding a fire. Looking at the swing of just Bheem, we can approximate him as a simple pendulum with a period of motion of 5.59 s. How long is the pendulum ? When Bheem swings, he goes a full distance, from side to side, of 10.2 m. What is his maximum velocity? What is his maximum acceleration?arrow_forwardThe position of a 0.300 kg object attached to a spring is described by x=0.271 m ⋅ cos(0.512π⋅rad/s ⋅t) (Assume t is in seconds.) Find the amplitude of the motion. Find the spring constant. Find the position of the object at t = 0.324 s. Find the object's velocity at t = 0.324 s.arrow_forward
- Min Min is hanging from her spring-arms off the edge of the level. Due to the spring like nature of her arms she is bouncing up and down in simple harmonic motion with a maximum displacement from equilibrium of 0.118 m. The spring constant of Min-Min’s arms is 9560. N/m and she has a mass of 87.5 kg. What is the period at which she oscillates? Find her maximum speed. Find her speed when she is located 5.00 cm from her equilibrium position.arrow_forward(a) What magnification in multiples is produced by a 0.150 cm focal length microscope objective that is 0.160 cm from the object being viewed? 15.9 (b) What is the overall magnification in multiples if an eyepiece that produces a magnification of 7.90x is used? 126 × ×arrow_forwardGravitational Potential Energyarrow_forward
- E = кедо Xo A continuous line of charge lies along the x axis, extending from x = +x to positive infinity. The line carries positive charge with a uniform linear charge density 10. (a) What is the magnitude of the electric field at the origin? (Use the following as necessary: 10, Xo, and ke.) (b) What is the direction of the electric field at the origin? O O O O O O G -y +z ○ -z +x -x +yarrow_forwardInclude free body diagramarrow_forward2 Spring 2025 -03 PITT Calculate the acceleration of a skier heading down a 10.0° slope, assuming the coefficient of cold coast at a constant velocity. You can neglect air resistance in both parts. friction for waxed wood on wet snow fly 0.1 (b) Find the angle of the slope down which this skier Given: 9 = ? 8=10° 4=0.1arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





