
Concept explainers
A vessel contains 1.00 × 104 oxygen molecules at 500 K. (a) Make an accurate graph of the Maxwell speed distribution function versus speed with points at speed intervals of 100 m/s. (b) Determine the most probable speed from this graph. (c) Calculate the average and rms speeds for the molecules and label these points on your graph. (d) From the graph, estimate the fraction of molecules with speeds in the range 300 m/s to 600 m/s.
(a)
The graph of the Maxwell speed distribution function versus speed with points at speed intervals of
Answer to Problem 38AP
The of the Maxwell speed distribution function versus speed with points at speed intervals of
Explanation of Solution
The Maxwell distribution curve is the graph between the distribution of speed and the change in speed or speed interval.
The number of molecules of oxygen in vessel is
Write the expression of Maxwell’s speed distribution function.
Here,
The mass of the molecules of oxygen
Here,
The molecular mass of the oxygen molecules in
Substitute
Substitute
Substitute the values of
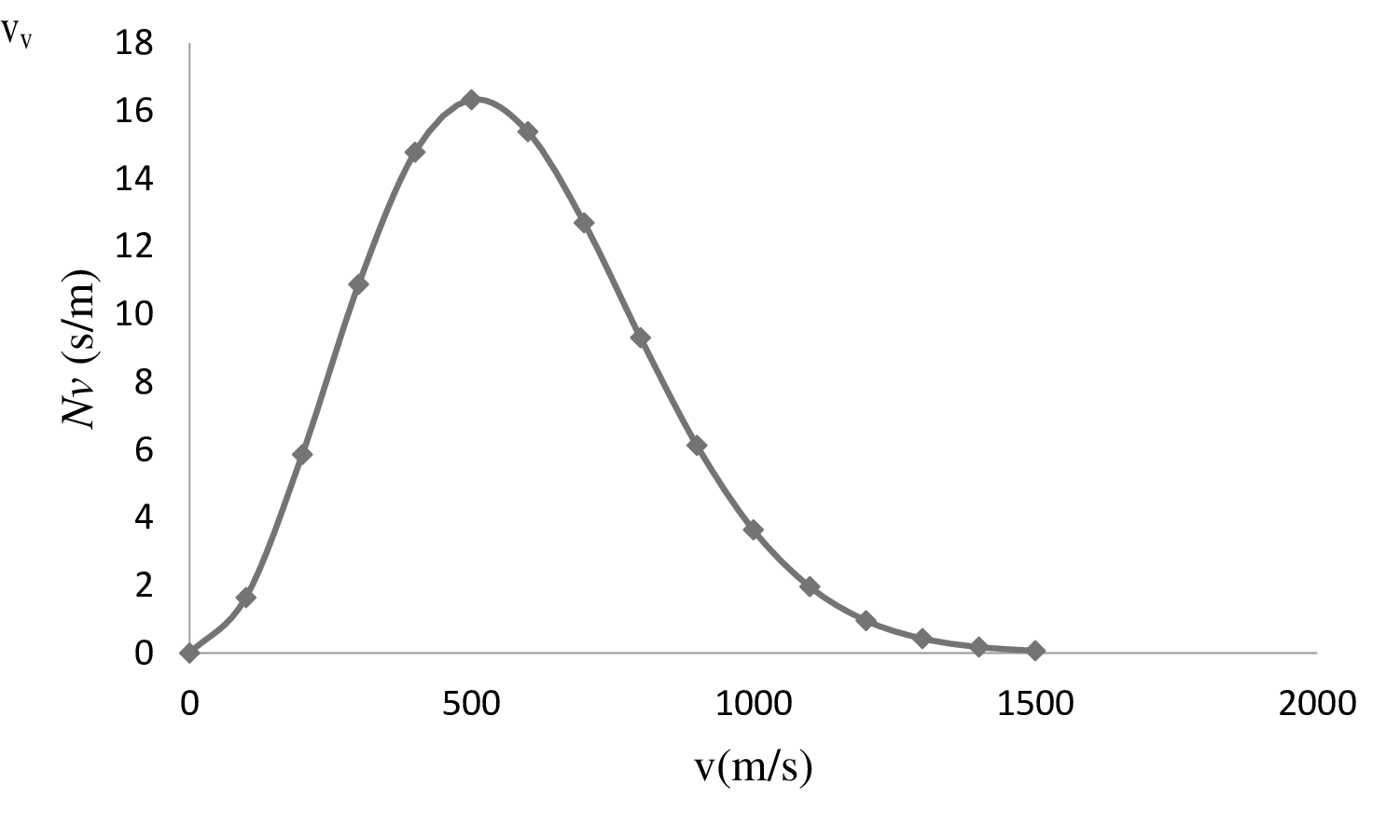
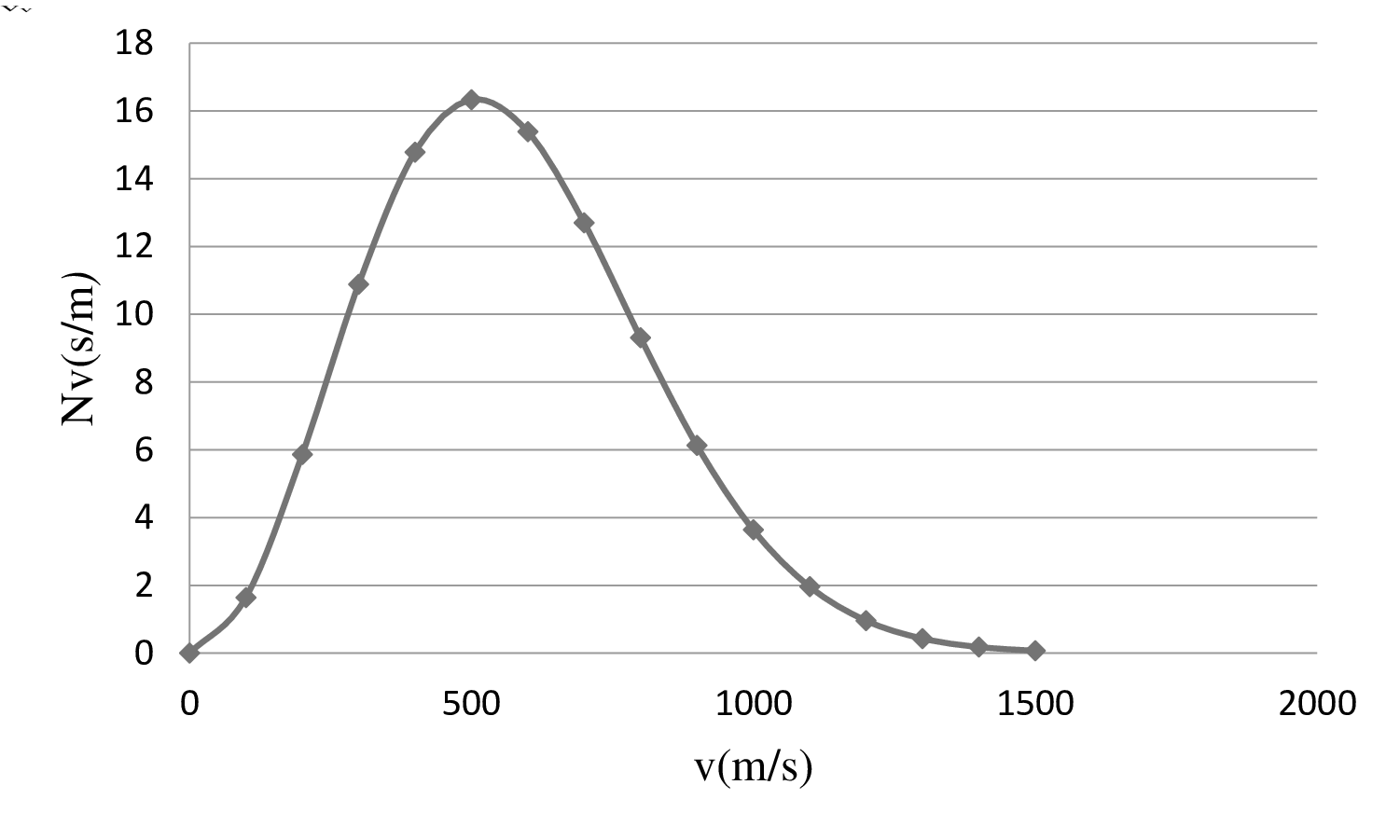
| 0 | 0 |
| 100 | 1.64 |
| 200 | 5.86 |
| 300 | 10.88 |
| 400 | 14.78 |
| 500 | 16.33 |
| 600 | 15.39 |
| 700 | 12.7 |
| 800 | 9.31 |
| 900 | 6.13 |
| 1000 | 3.64 |
| 1100 | 1.961 |
| 1200 | 0.96 |
| 1300 | 0.43 |
| 1400 | 0.18 |
| 1500 | 0.07 |
On the basis of the table, a graph is plotted below;
(b)
The most probable speed from the graph.
Answer to Problem 38AP
The most probable speed is
Explanation of Solution
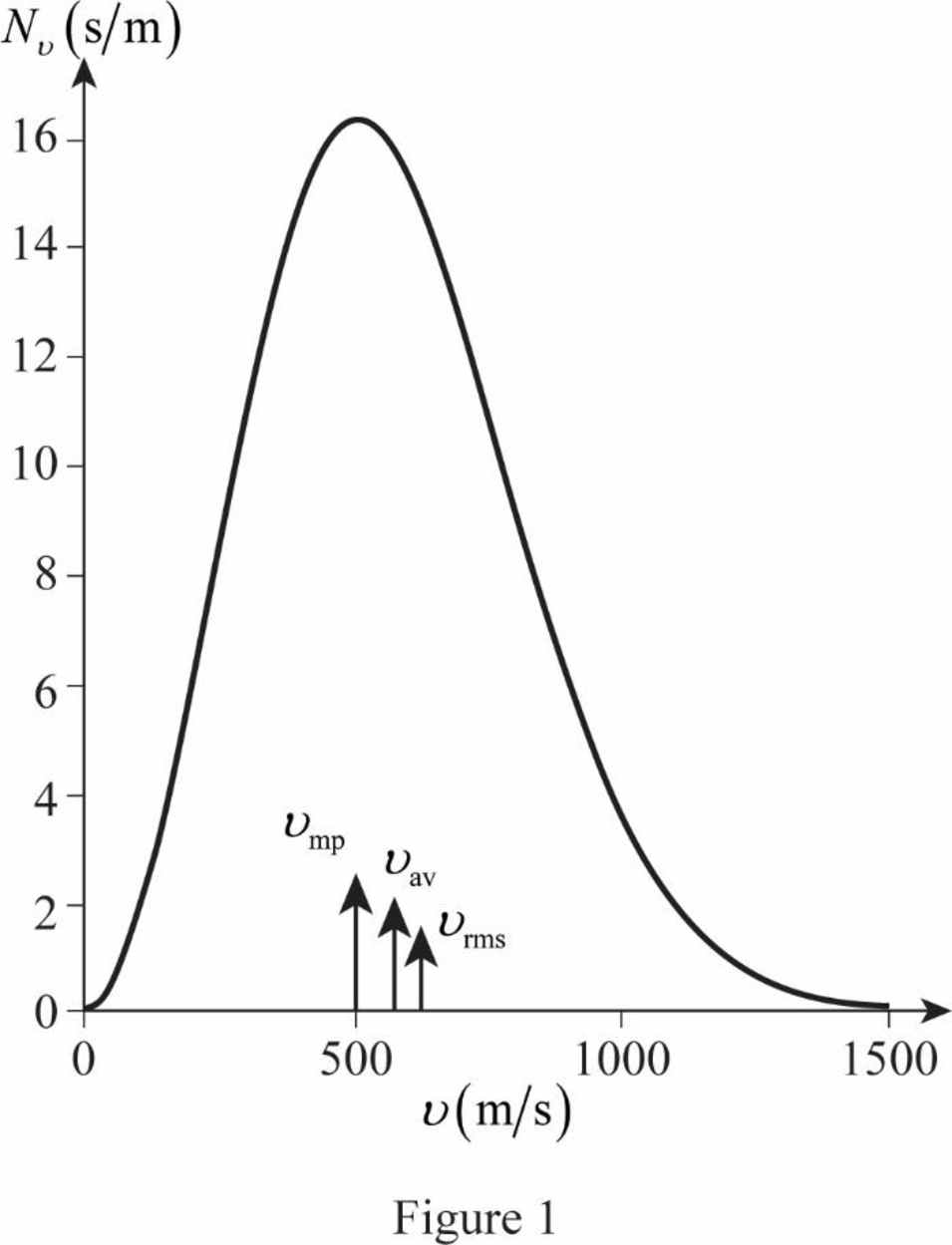
The most probable speed occurs where
Conclusion:
Therefore, the most probable speed is
(c)
The average and rms speeds for the molecules and label these points on the graph.
Answer to Problem 38AP
The average and rms speeds for the molecules is
Explanation of Solution
Write the expression of average velocity.
The mass of the molecules of oxygen
Substitute
The molecular mass of the oxygen molecules in
Substitute
Thus, the average speed is
Write the expression of rms velocity.
Substitute
Substitute
Thus, the rms velocity of the oxygen molecules is
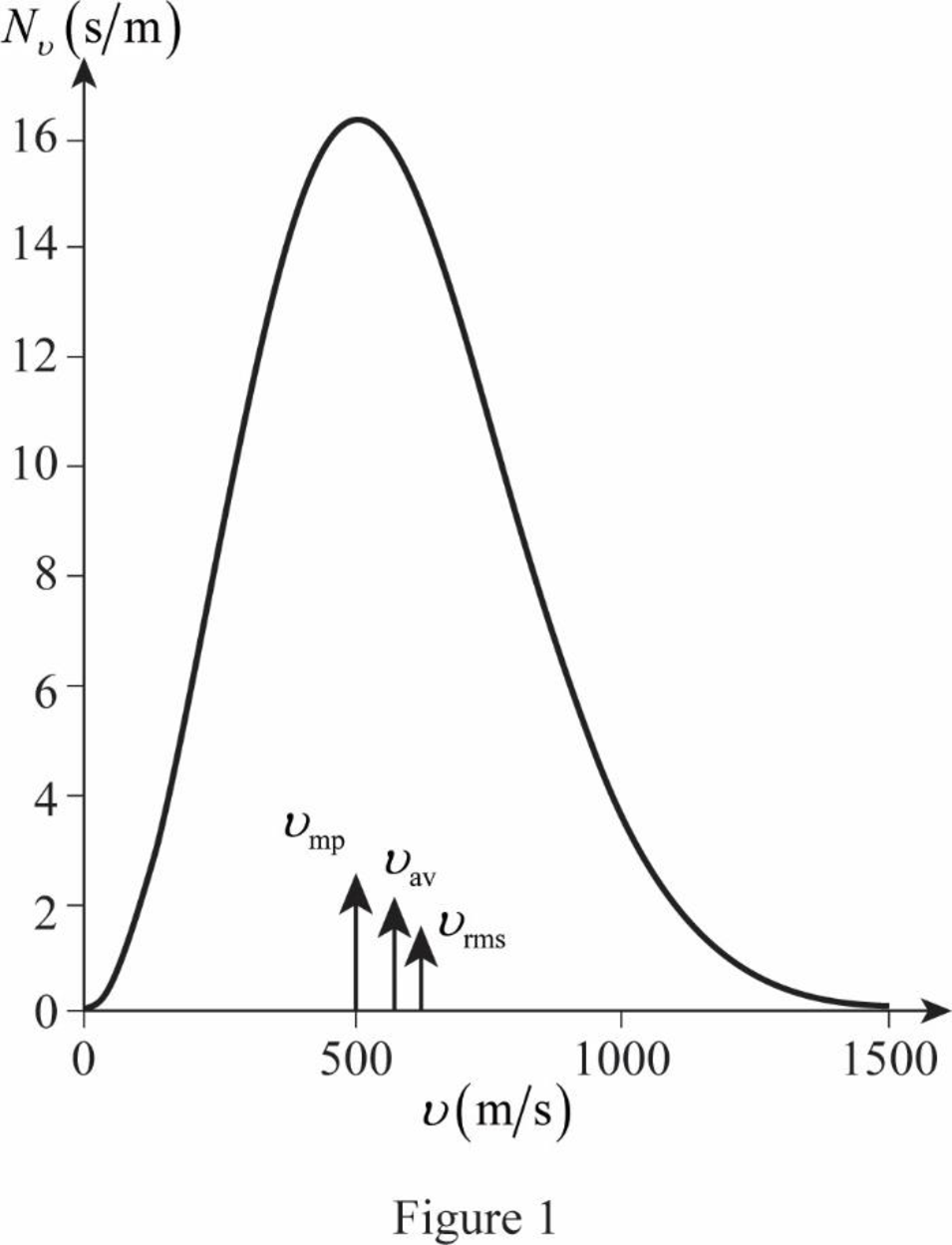
The graph of Maxwell’s curve is shown below;
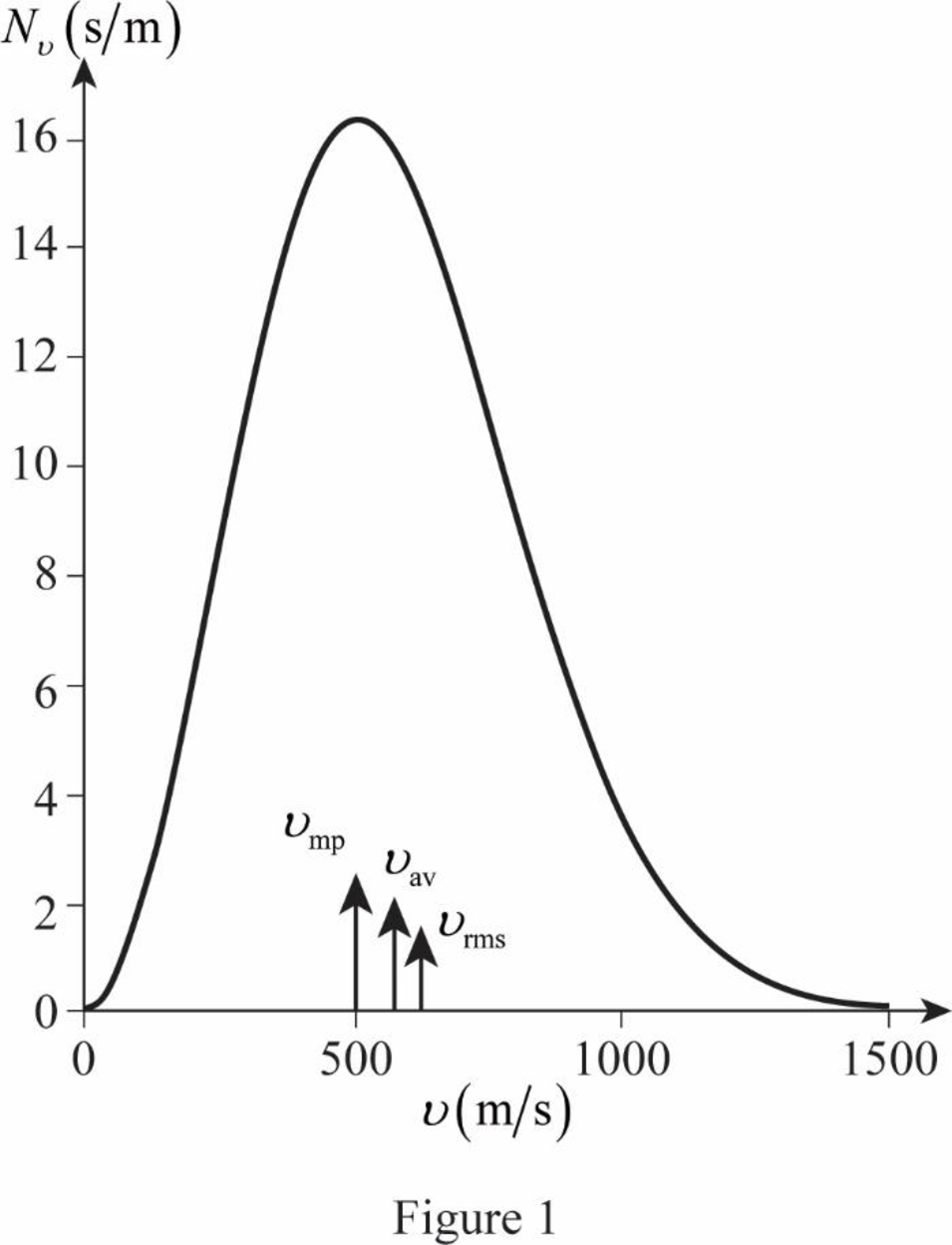
The point
Conclusion:
Therefore, the average and rms speeds for the molecules is
(d)
The fraction of molecules with the speed in the range of
Answer to Problem 38AP
The fraction of molecules with the speed in the range of
Explanation of Solution
The figure given below shows the Maxwell’s curve,
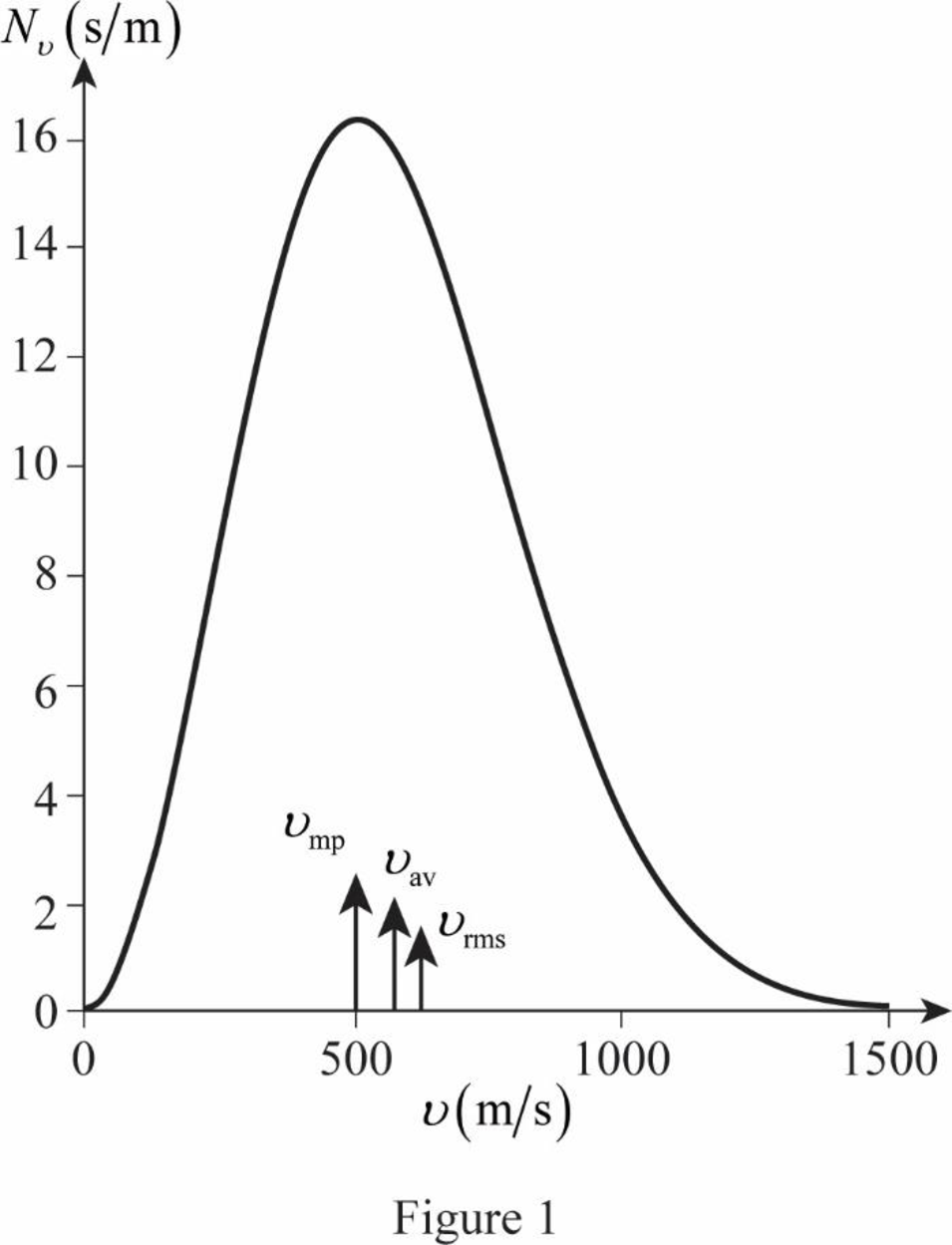
The area under the distribution curve in the range
Conclusion:
Write the area under the curve in the range
Therefore, the fraction of molecules with the speed in the range of
Want to see more full solutions like this?
Chapter 20 Solutions
PHYSICS:F/SCI.+ENGRS.,V.1
- 20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?arrow_forwardpls help on allarrow_forwardpls help on thesearrow_forward
- pls help on all asked questions kindlyarrow_forwardpls help on all asked questions kindlyarrow_forward19. Mount Everest, Earth's highest mountain above sea level, has a peak of 8849 m above sea level. Assume that sea level defines the height of Earth's surface. (re = 6.38 × 106 m, ME = 5.98 × 1024 kg, G = 6.67 × 10 -11 Nm²/kg²) a. Calculate the strength of Earth's gravitational field at a point at the peak of Mount Everest. b. What is the ratio of the strength of Earth's gravitational field at a point 644416m below the surface of the Earth to a point at the top of Mount Everest? C. A tourist watching the sunrise on top of Mount Everest observes a satellite orbiting Earth at an altitude 3580 km above his position. Determine the speed of the satellite.arrow_forward
- pls help on allarrow_forwardpls help on allarrow_forward6. As the distance between two charges decreases, the magnitude of the electric potential energy of the two-charge system: a) Always increases b) Always decreases c) Increases if the charges have the same sign, decreases if they have the opposite signs d) Increases if the charges have the opposite sign, decreases if they have the same sign 7. To analyze the motion of an elastic collision between two charged particles we use conservation of & a) Energy, Velocity b) Momentum, Force c) Mass, Momentum d) Energy, Momentum e) Kinetic Energy, Potential Energyarrow_forward
- pls help on all asked questions kindlyarrow_forwardpls help on all asked questions kindlyarrow_forward17. Two charges, one of charge +2.5 × 10-5 C and the other of charge +3.7 × 10-6 C, are 25.0 cm apart. The +2.5 × 10−5 C charge is to the left of the +3.7 × 10−6 C charge. a. Draw a diagram showing the point charges and label a point Y that is 20.0 cm to the left of the +3.7 × 10-6 C charge, on the line connecting the charges. (Field lines do not need to be drawn.) b. Calculate the net electric field at point Y.arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





