
Fundamentals of Physics
10th Edition
ISBN: 9781118230718
Author: David Halliday
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 34P
GO An ideal gas (1.0 mol) is the working substance in an engine that operates on the cycle shown in Fig. 20-30, Processes BC and DA are reversible and adiabatic. (a) Is the gas monatomic, diatomic, or polyatomic? (b) What is the engine efficiency?
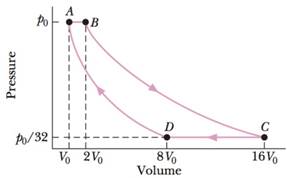
Figure 20-23 Problem 34.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An amoeba is 0.309 cm away from the 0.304 cm focal length objective lens of a microscope.
Two resistors of resistances R1 and R2, with R2>R1, are connected to a voltage source with voltage V0. When the resistors are connected in series, the current is Is. When the resistors are connected in parallel, the current Ip from the source is equal to 10Is. Let r be the ratio R1/R2. Find r. I know you have to find the equations for V for both situations and relate them, I'm just struggling to do so. Please explain all steps, thank you.
Bheem and Ram, jump off either side of a bridge while holding opposite ends of a rope and swing back and forth under the bridge to save a child while avoiding a fire. Looking at the swing of just Bheem, we can approximate him as a simple pendulum with a period of motion of 5.59 s. How long is the pendulum ? When Bheem swings, he goes a full distance, from side to side, of 10.2 m. What is his maximum velocity? What is his maximum acceleration?
Chapter 20 Solutions
Fundamentals of Physics
Ch. 20 - Point i in Fig. 20-19 represents the initial state...Ch. 20 - In lour experiments, blocks A and B, starting ill...Ch. 20 - A gas, confined to an insulated cylinder, is...Ch. 20 - An ideal monatomic gas at initial temperature T0...Ch. 20 - In four experiments, 2.5 mol of hydrogen gas...Ch. 20 - A box contains 100 atoms in a configuration that...Ch. 20 - Does the entropy per cycle increase, decrease, or...Ch. 20 - Three Carnot engines operate between temperature...Ch. 20 - An inventor claims to have invented four engines,...Ch. 20 - Does the entropy per cycle increase, decrease, or...
Ch. 20 - SSM Suppose 4.00 mol of an ideal gas undergoes a...Ch. 20 - An ideal gas undergoes a reversible isothermal...Ch. 20 - ILW A 2.50 mol sample of an ideal gas expands...Ch. 20 - How much energy must be transferred as heat for a...Ch. 20 - ILW Find a the energy absorbed as heat and b the...Ch. 20 - a What is the entropy change of a 12.0 g ice cube...Ch. 20 - ILW A 50.0 g block of copper whose temperature is...Ch. 20 - At very low temperatures, the molar specific heat...Ch. 20 - A 10 g ice cube at 10oC is placed in a lake whose...Ch. 20 - A 364 g block is put in contact with a thermal...Ch. 20 - SSM WWW In an experiment, 200 g of aluminum with a...Ch. 20 - A gas sample undergoes a reversible isothermal...Ch. 20 - In the irreversible process of Fig. 20-5, let the...Ch. 20 - Prob. 14PCh. 20 - A mixture of 1773 g of water and 227 g of ice is...Ch. 20 - GO An 8.0 g ice cube at -10C is put into a Thermos...Ch. 20 - Prob. 17PCh. 20 - GO A 2.0 mol sample of an ideal monatomic gas...Ch. 20 - Suppose 1.00 mol of a monatomic ideal gas is taken...Ch. 20 - Expand 1.00 mol of an monatomic gas initially at...Ch. 20 - GO Energy can be removed from water as heat at and...Ch. 20 - GO An insulated Thermos contains 130 g of water at...Ch. 20 - A Carnot engine whose low-temperature reservoir is...Ch. 20 - A Carnot engine absorbs 52 kJ as heat and exhausts...Ch. 20 - A Carnot engine has an efficiency of 22.0. It...Ch. 20 - In a hypothetical nuclear fusion reactor, the fuel...Ch. 20 - SSM WWW A Carnot engine operates between 235C and...Ch. 20 - In the first stage of a two-stage Carnot engine,...Ch. 20 - GO Figure 20-27 shows a reversible cycle through...Ch. 20 - A 500 W Carnot engine operates between...Ch. 20 - The efficiency of a particular car engine is 25...Ch. 20 - GO A Carnot engine is set up to produce a certain...Ch. 20 - SSM ILW Figure 20-29 shows a reversible cycle...Ch. 20 - GO An ideal gas 1.0 mol is the working substance...Ch. 20 - The cycle in Fig. 20-31 represents the operation...Ch. 20 - How much work must be done by a Carnot...Ch. 20 - SSM A heat pump is used to heal a building, The...Ch. 20 - The electric motor of a heat pump transfers energy...Ch. 20 - SSM A Carnot air conditioner lakes energy from the...Ch. 20 - To make ice, a freezer that is a reverse Carnot...Ch. 20 - ILW An air conditioner operating between 93F and...Ch. 20 - The motor in a refrigerator has a power of 200 W....Ch. 20 - GO Figure 20-32 represents a Carnot engine that...Ch. 20 - a During each cycle, a Carnot engine absorbs 750 J...Ch. 20 - Prob. 45PCh. 20 - A box contains N identical gas molecules equally...Ch. 20 - SSM WWW A box contains N gas molecules, Consider...Ch. 20 - Four particles are in the insulated box of Fig....Ch. 20 - A cylindrical copper rod of length 1.50 m and...Ch. 20 - Suppose 0.550 mol of an ideal gas is isothermally...Ch. 20 - Prob. 51PCh. 20 - Suppose 1.0 mol of a monatomic ideal gas initially...Ch. 20 - GO Suppose that a deep shaft were drilled in...Ch. 20 - What is the entropy change for 3.20 mol of an...Ch. 20 - A 600 g lump of copper at 80.0C is placed in 70.0...Ch. 20 - Figure 20-33 gives the force magnitude F versus...Ch. 20 - The temperature of 1.00 mol of a monatomic ideal...Ch. 20 - Repeat Problem 57, with the pressure now kept...Ch. 20 - SSM A 0.600 kg sample of water is initially ice at...Ch. 20 - A three-step cycle is undergone by 3.4 mol of an...Ch. 20 - An inventor has built an engine X and claims that...Ch. 20 - Suppose 2.00 mol of a diatomic gas is taken...Ch. 20 - A three-step cycle is undergone reversibly by 4.00...Ch. 20 - a A Carnot engine operates between a hot reservoir...Ch. 20 - A 2.00 mol diatomic gas initially at 300 K...Ch. 20 - An ideal refrigerator does 150 J of work to remove...Ch. 20 - Suppose that 260 J is conducted from a...Ch. 20 - An apparatus that liquefies helium is in a room...Ch. 20 - GO A brass rod is in thermal contact with a...Ch. 20 - A 45.0 g block of tungsten at 30.0C and a 25.0 g...Ch. 20 - Prob. 71PCh. 20 - Calculate the efficiency of a fossil-fuel power...Ch. 20 - SSM A Carnot refrigerator extracts 35.0 kJ as heat...Ch. 20 - A Carnot engine whose high-temperature reservoir...Ch. 20 - SSM System A of three particles and system B of...Ch. 20 - Figure 20-36 shows a Carnot cycle on a T-S...Ch. 20 - Find the relation between the efficiency of a...Ch. 20 - A Carnot engine has a power of 500 W. It operates...Ch. 20 - In a real refrigerator, the low-temperature coils...
Additional Science Textbook Solutions
Find more solutions based on key concepts
61. What is the pH of a solution in which 224 mL of HCl(g), measured at 27.2 °C and 1.02 atm, is dissolved in 1...
Chemistry: A Molecular Approach (4th Edition)
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
All of the following processes are involved in the carbon cycle except: a. photosynthesis b. cell respiration c...
Human Biology: Concepts and Current Issues (8th Edition)
12.1 Give the IUPAC name for each of the following:
a. CH3-CH2-OH
b.
c.
d.
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Checkpoint 24:
How is DNA packed in the nucleus?
Principles of Anatomy and Physiology
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The position of a 0.300 kg object attached to a spring is described by x=0.271 m ⋅ cos(0.512π⋅rad/s ⋅t) (Assume t is in seconds.) Find the amplitude of the motion. Find the spring constant. Find the position of the object at t = 0.324 s. Find the object's velocity at t = 0.324 s.arrow_forwardMin Min is hanging from her spring-arms off the edge of the level. Due to the spring like nature of her arms she is bouncing up and down in simple harmonic motion with a maximum displacement from equilibrium of 0.118 m. The spring constant of Min-Min’s arms is 9560. N/m and she has a mass of 87.5 kg. What is the period at which she oscillates? Find her maximum speed. Find her speed when she is located 5.00 cm from her equilibrium position.arrow_forward(a) What magnification in multiples is produced by a 0.150 cm focal length microscope objective that is 0.160 cm from the object being viewed? 15.9 (b) What is the overall magnification in multiples if an eyepiece that produces a magnification of 7.90x is used? 126 × ×arrow_forward
- Gravitational Potential Energyarrow_forwardE = кедо Xo A continuous line of charge lies along the x axis, extending from x = +x to positive infinity. The line carries positive charge with a uniform linear charge density 10. (a) What is the magnitude of the electric field at the origin? (Use the following as necessary: 10, Xo, and ke.) (b) What is the direction of the electric field at the origin? O O O O O O G -y +z ○ -z +x -x +yarrow_forwardInclude free body diagramarrow_forward
- 2 Spring 2025 -03 PITT Calculate the acceleration of a skier heading down a 10.0° slope, assuming the coefficient of cold coast at a constant velocity. You can neglect air resistance in both parts. friction for waxed wood on wet snow fly 0.1 (b) Find the angle of the slope down which this skier Given: 9 = ? 8=10° 4=0.1arrow_forwarddry 5. (a) When rebuilding her car's engine, a physics major must exert 300 N of force to insert a c piston into a steel cylinder. What is the normal force between the piston and cyli=030 What force would she have to exert if the steel parts were oiled? k F = 306N 2 =0.03 (arrow_forwardInclude free body diagramarrow_forward
- Include free body diagramarrow_forwardTest 2 МК 02 5. (a) When rebuilding her car's engine, a physics major must exert 300 N of force to insert a dry = 0.03 (15 pts) piston into a steel cylinder. What is the normal force between the piston and cylinder? What force would she have to exert if the steel parts were oiled? Mk Giren F = 306N MK-0.3 UK = 0.03 NF = ?arrow_forward2. A powerful motorcycle can produce an acceleration of 3.50 m/s² while traveling at 90.0 km/h. At that speed the forces resisting motion, including friction and air resistance, total 400 N. (Air resistance is analogous to air friction. It always opposes the motion of an object.) What force does the motorcycle exert backward on the ground to produce its acceleration if the mass of the motorcycle with rider is 245 ke? a = 350 m/s 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY