
(a)
Interpretation:
6-phosphoglucono-⏹-lactone should be identified.
Concept introduction:
6-phosphoglucono-⏹-lactone is formed during the pentose phosphate pathway. In the first step of pentose phosphate pathway, dehydrogenation of glucose-6-phosphate at C-1 takes produce 6-phosphoglucono-⏹-lactone.
Answer to Problem 28P
The compound C in the reaction is 6-phosphoglucono-⏹-lactone.
Explanation of Solution
6-phosphoglucono-⏹-lactone is an intramolecular ester formed by the reaction of C-1 carboxyl group and C-5 hydroxyl group. It is formed by dehydrogenation of C-1 carbon of Glucose-6-phosphate. Hydroxyl group at C-1 of glucose-6-phosphate is converted to carbonyl group. Therefore, the structure of 6-phosphoglucono-⏹-lactone is (C).
(b)
Interpretation:
The reactions producing NADPH should be determined.
Concept introduction:
Two molecules of NADPH are produced duringthe oxidative phase of pentose phosphate pathway.
Answer to Problem 28P
The reactions B and F produce NADPH.
Explanation of Solution
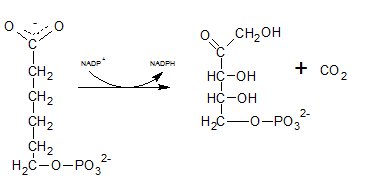
First NADPH is produced when the C-1 in glucose-6-phosphate is dehydrogenated into 6-phosphoglucono-⏹-lactone by glucose-6-phosphate dehydrogenase. This 6-phosphoglucono-⏹-lactone is hydrolyzed by a lactonase resulting 6-phosphogluconate. This 6C sugar acid is then decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate. In this step also NADP+ acts as the electron acceptor and produce NADPH.
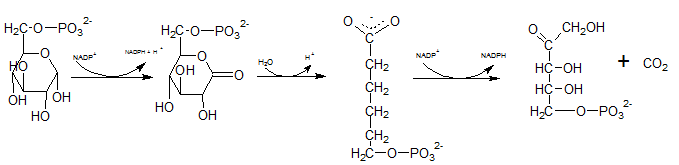
Therefore,reactions B and F produces NADPH.
(c)
Interpretation:
Ribulose-5-phosphate should be identified.
Concept introduction:
Ribulose-5-phosphate is the productof pentose phosphate pathway.
Answer to Problem 28P
The compound G in the reaction is Ribulose-5-phosphate.
Explanation of Solution
As the first step of oxidative phase of pentose phosphate pathway, C-1 of glucose-6-phosphate is dehydrogenated into 6-phosphoglucono-⏹-lactone by glucose-6-phosphate dehydrogenase. Then this 6-phosphoglucono-⏹-lactone is hydrolyzed by a lactonase resulting 6-phosphogluconate. This 6 C sugar acid is then decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate.
Therefore, Gis Ribulose-5-phosphate.
(d)
Interpretation:
The CO2generating reaction should be determined.
Concept introduction:
The decarboxylation reactions generate CO2 as a by-product.
Answer to Problem 28P
The reactions F produce CO2.
Explanation of Solution
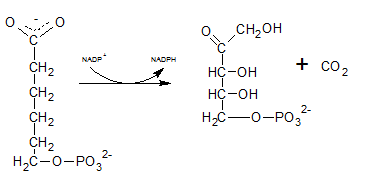
The six C sugar acid, 6-phosphogluconate formed during pentose phosphate pathway is oxidatively decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate. The final product is a five-carbon sugar, and release CO2.
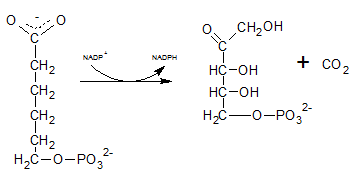
So. the reaction F produce CO2.
(e)
Interpretation:
6-phosphogluconate should be identified.
Concept introduction:
6-phosphogluconate is a 6C sugar acid which forms during pentose phosphate pathway.
Answer to Problem 28P
The compound E in the reaction is 6-phosphogluconate.
Explanation of Solution
In the first step of the oxidative phase of pentose phosphate pathway, C-1 of glucose-6-phosphate is dehydrogenated into 6-phosphoglucono-⏹-lactone by glucose-6-phosphate dehydrogenase which is hydrolyzed by a lactonase resulting 6-phosphogluconate.
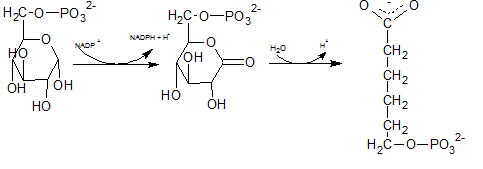
The end product of above reaction is 6-phosphogluconate. Thus the compound E in the reaction is 6-phosphogluconate.
(f)
Interpretation:
The reaction that is catalyzed by phosphopentose isomerase should be determined.
Concept introduction:
Phosphopentose isomerase is an enzyme which involves in isomerization reaction.
Answer to Problem 28P
The reactions H uses Phosphopentose isomerase enzyme.
Explanation of Solution
Ribulose-5-phosphate is isomerized to ribose-5-phosphate by phosphopentose isomerase. The enzyme, phosphopentose isomerase catalyze the conversion of a ketose sugar (Ribulose-5-phosphate ) to an aldose sugar (ribose-5-phosphate).
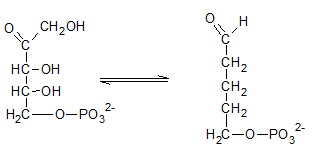
Therefore, the reaction H needs the enzyme phosphopentose isomerase.
(g)
Interpretation:
Ribose-5-phosphate should be identified.
Concept introduction:
Ribose-5-phosphate is the end product of the oxidative phase of pentose phosphate pathway.
Answer to Problem 28P
The compound I in the reaction is Ribose-5-phosphate.
Explanation of Solution
The ribulose-5-phosphate is obtained in pentose phosphate pathway when the ribose-5-phosphate is isomerized by phosphopentose isomerase.
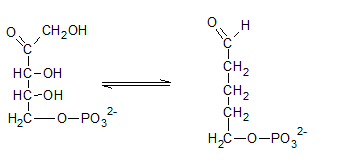
Therefore, the compound I in the reaction is Ribose-5-phosphate.
(h)
Interpretation:
Reaction catalyzed by lactonase should be determined.
Concept introduction:
Lactonases catalyzes the hydrolysis of ester bonds.
Answer to Problem 28P
The reactions D uses the enzymeLactonases.
Explanation of Solution
The degydrogenated product of glucose-6-phospahte is 6-phosphoglucono-⏹-lactone. This is a 6 membered ring structure and have an ester bond between C-1 carbonyl carbon and C-5 hydroxyl Oxygen. This bond is hydrolyzed by lactonase and to produce 6-phosphogluconate. The reaction is indicated by letter D.
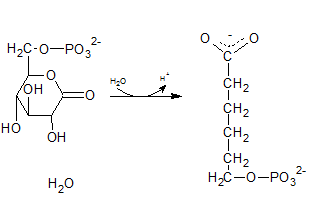
(i)
Interpretation:
Glucose-6-phosphate should be identified.
Concept introduction:
The pentose phosphate pathway is initiated by the oxidation of glucose-6-phosphate.
Answer to Problem 28P
The compound A in the reaction is glucose-6-phosphate.
Explanation of Solution
Glucose-6-phosphate is a 6-carbon sugar and have a ring structure where the hydroxyl group at C-6 is phosphorylated.
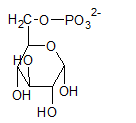
The compound A in the reaction is glucose-6-phosphate.
(j)
Interpretation:
The reaction catalyzed by 6-phosphogluconate dehydrogenase should be determined.
Concept introduction:
Dehydrogenases are the enzymes which catalyzes the removal of hydrogen molecules with the help of coenzymes NAD and FAD.
Answer to Problem 28P
The reactions F uses the enzymedehydrogenases.
Explanation of Solution
6-phosphogluconate is oxidatively decarboxylated by 6-phosphogluconate dehydrogenase into ribulose-5-phosphate which is indicated by reaction F.
(k)
Interpretation:
Reaction that is catalyzed by glucose-6-phosphate dehydrogenase should be determined.
Concept introduction:
In the first reaction of pentose phosphate pathway glucose-6-phosphate is dehydrogenated to produce phosphoglucono-⏹-lactone.
Answer to Problem 28P
The reactions B uses the enzymeglucose-6-phosphate dehydrogenases.
Explanation of Solution
In the first step of pentose phosphate pathway, Glucose-6-phosphate is dehydrogenated at C-1 by glucose-6-phosphate dehydrogenase enzyme into 6-phosphoglucono-⏹-lactone.
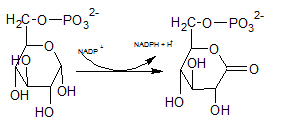
So, the reactions B uses the enzymeglucose-6-phosphate dehydrogenases.
Want to see more full solutions like this?
Chapter 20 Solutions
BIOCHEMISTRY W/1 TERM ACHEIVE ACCESS
- Classify the monosaccharides. H-C-OH H. H-C-OH H-C-OH CH₂OH H-C-OH H-C-OH H-C-OH CH₂OH CH₂OH CH₂OH CH₂OH D-erythrose D-ribose D-glyceraldehyde Dihydroxyacetone CH₂OH CH₂OH C=O Answer Bank CH₂OH C=0 HO C-H C=O H-C-OH H-C-OH pentose hexose tetrose H-C-OH H-C-OH H-C-OH aldose triose ketose CH₂OH CH₂OH CH₂OH D-erythrulose D-ribulose D-fructosearrow_forwardFatty acids are carboxylic acids with long hydrophobic tails. Draw the line-bond structure of cis-A9-hexadecenoate. Clearly show the cis-trans stereochemistry.arrow_forwardThe formation of acetyl-CoA from acetate is an ATP-driven reaction: Acetate + ATP + COA Acetyl CoA+AMP+ PP Calculate AG for this reaction given that the AG for the hydrolysis of acetyl CoA to acetate and CoA is -31.4 kJ mol-1 (-7.5 kcal mol-¹) and that the AG for hydrolysis of ATP to AMP and PP; is -45.6 kJ mol-1 (-10.9 kcal mol-¹). AG reaction kJ mol-1 The PP, formed in the preceding reaction is rapidly hydrolyzed in vivo because of the ubiquity of inorganic pyrophosphatase. The AG for the hydrolysis of pyrophosphate (PP.) is -19.2 KJ mol-¹ (-4.665 kcal mol-¹). Calculate the AG° for the overall reaction, including pyrophosphate hydrolysis. AGO reaction with PP, hydrolysis = What effect does the presence of pyrophosphatase have on the formation of acetyl CoA? It does not affect the overall reaction. It makes the overall reaction even more endergonic. It brings the overall reaction closer to equilibrium. It makes the overall reaction even more exergonic. kJ mol-1arrow_forward
- Consider the Haworth projections of ẞ-L-galactose and ẞ-L-glucose shown here. OH CH₂OH OH CH₂OH OH OH OH ОН OH он B-L-galactose B-L-glucose Which terms describe the relationship between these two sugars? epimers enantiomers anomers diastereomersarrow_forwardClassify each characteristic as describing anabolism or catabolism. Anabolism Answer Bank Catabolism transforms fuels into cellular energy, such as ATP or ion gradients uses NADPH as the electron carrier synthesizes macromolecules requires energy inputs, such as ATP uses NAD+ as the electron carrier breaks down macromoleculesarrow_forwardThe table lists the standard free energies (AG") of hydrolysis of some phosphorylated compounds. Compound kJ mol-1 kcal mol-1 Phosphoenolpyruvate (PEP) -61.9 -14.8 1,3-Bisphosphoglycerate (1,3-BPG) -49.4 -11.8 Creatine phosphate -43.1 -10.3 ATP (to ADP) -30.5 -7.3 Glucose 1-phosphate -20.9 -5.0 Pyrophosphate (PP) -19.3 -4.6 Glucose 6-phosphate -13.8 -3.3 Glycerol 3-phosphate -9.2 -2.2 What is the direction of each of the reactions shown when the reactants are initially present in equimolar amounts? (a) ATP + H2O ADP + P (b) ATP + glycerol glycerol 3-phosphate + ADParrow_forward
- Characterize each term or phrase as pertaining to simple or facilitated diffusion. Simple diffusion Facilitated diffusion Answer Bank requires an input of free energy lipophilic molecules directly through membrane via channels polar molecules Na+arrow_forwardSort the descriptions into properties that describe either saturated phospholipids or unsaturated phospholipids. Saturated phospholipids Saturated and unsaturated phospholipids Unsaturated phospholipids Answer Bank have no double bonds in the fatty acid carbon chains have straight fatty acid tails have at least one double bond in the fatty acid tails have bent fatty acid tails are built upon a glycerol backbone make the membrane somewhat rigid at low temperatures allow the membrane to remain fluid and flexible at low temperatures fatty acid tails pack tightly together maintain some space between adjacent phospholipidsarrow_forwardPlace the events of an action potential in order, starting and ending with a cell at its resting membrane potential. Cell starts at its resting membrane potential. Cell returns to its resting membrane potential. Answer Bank K+ channels fully open, and Na+ channels are inactivated. K* rushes out of the cell, causing repolarization. K+ channels close slowly, resulting in hyperpolarization. Na+ channel gates reset. Fast Na+ and slow K+ channels are activated. Na rushes into the cell, causing membrane depolarization. Ligand activation of the acetylcholine receptor depolarizes the membrane.arrow_forward
- Glucose and fructose are reducing sugars. Sucrose, or table sugar, is a disaccharide consisting of both fructose and glucose. Is sucrose a reducing sugar? Why or why not? No, because only one anomeric carbon is involved in the glycosidic linkage. No, because both anomeric carbons are involved in the glycosidic linkage. Yes, because the fructose unit can convert to the open-chain form. Yes, because the glucose unit can convert to the open-chain form. Which statements about reducing sugars are true? The oxidation of a reducing sugar forms a carboxylic acid sugar. D-Arabinose (an aldose) is a reducing sugar. Reducing sugars contain keto groups instead of aldehyde groups. A disaccharide with its anomeric carbons joined by the glycosidic linkage cannot be a reducing sugar. A reducing sugar will not react with the Cu² + in Fehlings's reagent.arrow_forwardExamine the pairs of molecules and identify the more-reduced molecule in each pair. H-C- CH, OH CH HO-C-H CH₁₂ Pyruvate Ethanol Acetaldehyde Lactate COO H-C H H- -C-H COO- Succinate Fumarate -OOC COO H COO- H――000- CH₂ COO- Oxalosuccinate H-C-OH OOC-C-H CH₂ COO Isocitratearrow_forwardClassify each description as characterizing facilitated diffusion, primary active transport, secondary active transport, or both primary and secondary active transport. Facilitated diffusion Primary active transport Secondary active transport Primary and secondary active transport Answer Bank requires ATP includes lactose permease directly uses ATP hydrolysis to pump substances across the membrane includes the Na+-K+ ATPase pump always moves more than one substance at a time movement of substances against an electrochemical gradient does not require energy input includes uniporters uses energy stored in electrochemical gradients generated by pumpsarrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning





