
Concept explainers
We now continue the use of
To make your own roadmap for Chapters 20–23, take a blank sheet of paper and write the following
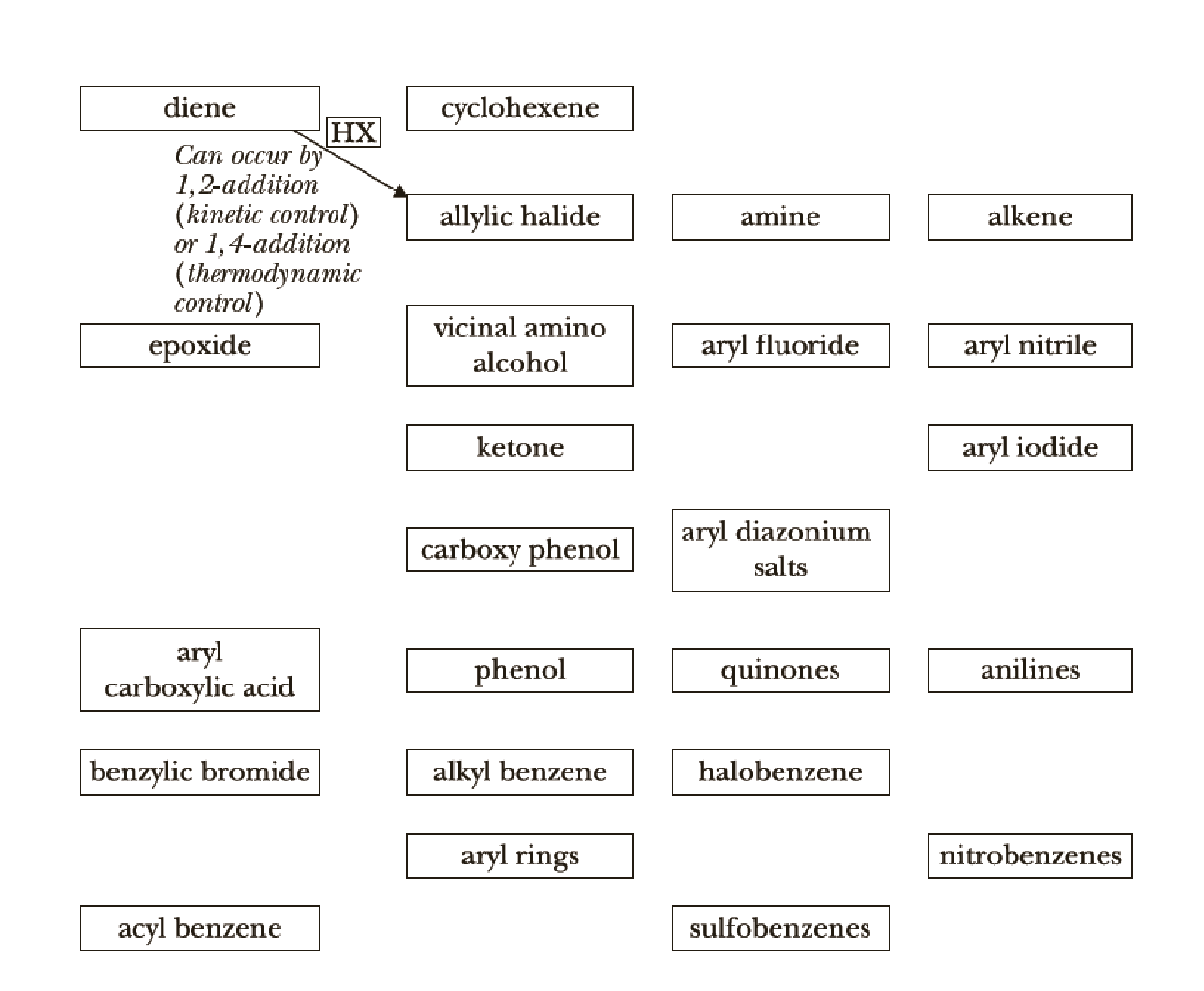
We now continue the use of organic chemistry reaction roadmaps. Because of the unique nature of the new reactions presented, we recommend that you make a new roadmap only for Chapters 20–23.
To make your own roadmap for Chapters 20–23, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized sheet of paper filled out in landscape orientation.
Interpretation:
A reaction roadmap has to be made for the reactions in the study Guide sections of chapters 20-23.
Concept Introduction:
Aromatic substitutions:
Aromatic substitution can be of two types, those are electrophilic and nucleophilic. Aromatic electrophilic substitution is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. For example nitration, sulphonation, acylation, alkylation, halogenation to aromatic ring is aromatic electrophilic substitution reaction.
Aromatic nucleophilic substitution reactions are those reactions where nucleophile replaces a good leaving group.
Sigmatropic reaction:
A sigmatropic reaction is a pericyclic reaction where the net result is change of one sigma bond to another sigma bond in an uncatalysed intramolecular process.
Explanation of Solution
The reaction road maps for the reactions in the study Guide section of chapters (20-23) will help us to find out the reactions in easy way. The section number indicates the respective reaction segments involved in the study guide section.
Chapters 20 roadmap reaction legend,
Reaction 20.1:
Electrophilic addition to conjugate diene:
Conjugated diene undergoes both 1,2-addition and 1,4-addition reactions with electrophiles often giving mixtures of both kinds of products. The ratio of 1,2-addition to 1,4-addition is temperature dependent. 1,2-addition products are kinetically controlled product and 1,4 addition products are thermodynamically controlled product. When a conjugated diene reacts with
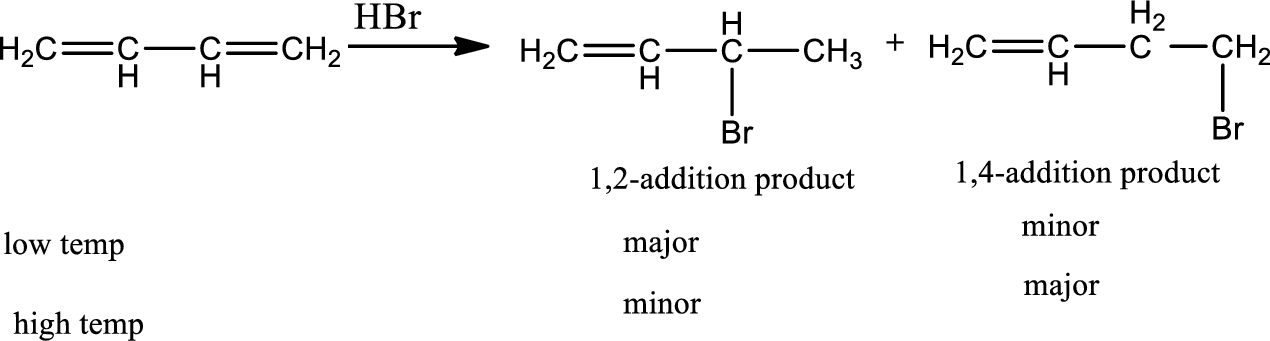
Reaction 20.2:
Diels-Alder reaction:
Conjugated dienes react with certain type of molecules having double or triple bonds, known as dienophile, to form two new sigma bond and a ring structure on the lose of pi bond in a reaction called Diels-Alder reaction. This reaction takes place in a concerted mechanism without intermediates and involves a redistribution of six pi electrons in a cyclic transition state where the pi bond breaking and new sigma bond making occurs simulteneously. The configuration of the diene and dienophile is preserved. Formation of the endo adduct is favoured. This reaction is facilitated by having electron withdrawing groups on dienophile and electron donating groups on diene. The diene must be on s-cis conformation to react. Diels-Alder reaction is an example of
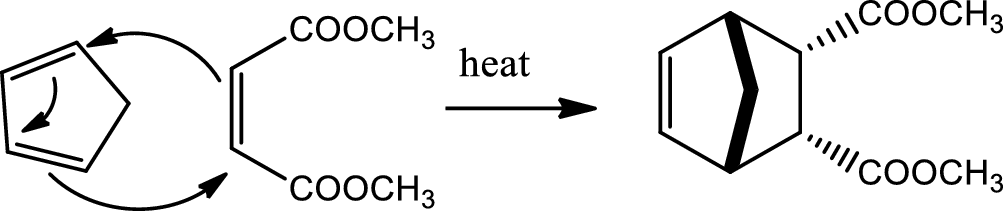
Reaction 20.3:
Claisen rearrangement:
The Claisen rearrangement is a powerful carbon-carbon bond forming chemical reaction. This rearrangement transforms an allyl phenyl ether to an ortho-substituted phenol. This rearrangement is an exothermic, concerted pericyclic reaction. The kinetics are first order and the whole transformation proceeds through a highly ordered cyclic transition state and it is intramolecular.
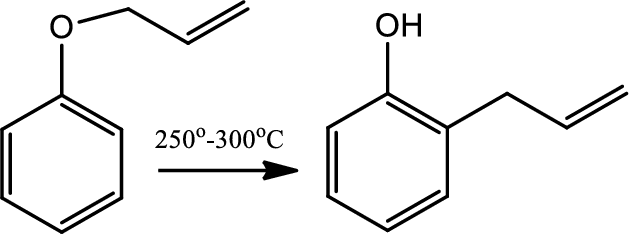
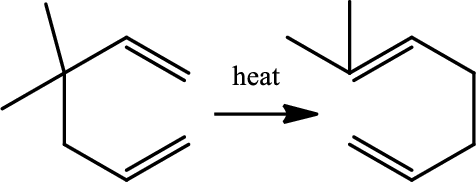
Reaction 20.4:
Cope rearrangement:
Cope rearrangement is the
Chapters 21 roadmap reaction legend,
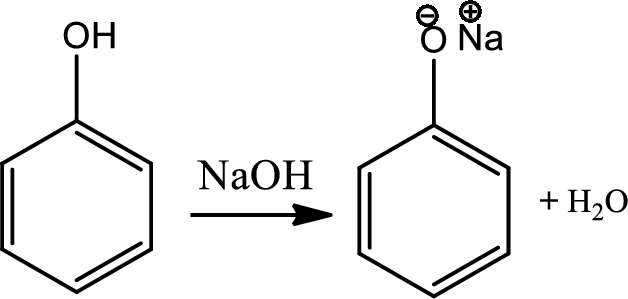
Reaction 21.1:
Acid base reaction of phenol:
Water soluble phenols react with the strong bases to give water soluble salts.
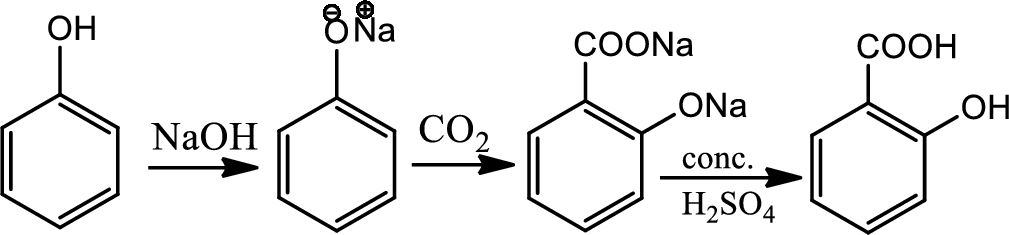
Reaction 21.2:
Kolbe synthesis:
Kolbe synthesis is a carboxylation chemical reaction that involves heating of sodim phenoxide with carbon dioxide and then treating the final product with sulfuric acid. This is basically nucleophilic addition of phenoxide to carbon dioxide that gives substituted cyclohexadienone which undergoes keto-enol tautomerism to regenerate aromatic ring. This gives maily ortho product which is known as salicylic acid.
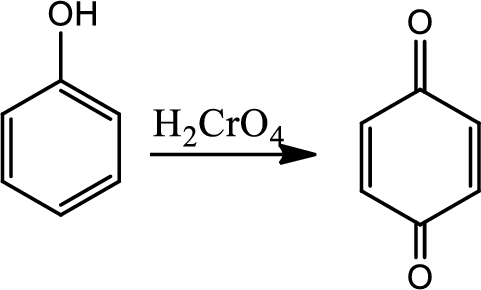
Reaction 21.3:
Oxidation of phenols to quinone:
Phenols can be easily oxidised to quinone. Oxidation of phenols by
Reaction 21.4:
Oxidation at benzylic position:
The benzylic carbon bonded hydrogen is weak and oxidation at benzylic position is to break the benzylic
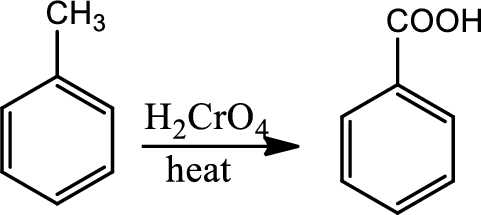
Reaction 21.5:
Halogenation at benzylic position:
Halogenation is regioselective for benzylic position and occurs by a radical chain mechanism. Bromination shows higher regioselectivity for a benzylic position than does chlorination. The reaction occurs via a radical chain mechanism which is initiated when the halohen molecule undergoes homolytic cleavage and gives two halide radical. One of the radical abstracts the benzylic hydrogen to create a resonance stabilised benzylic radical that reacts with another molecule of halogen to give the halogenated product and the new radical continues the chain reaction. NBS is used as the source of bromine.
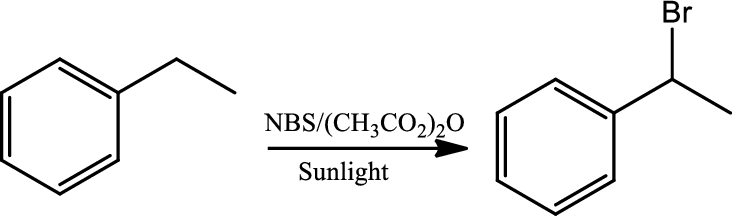
Reaction 21.6:
Hydrogenolysis of benzylic ethers:
Bezylic ethers can be cleaved to give an alcohol or toluene using catalytic hydrogenation of (
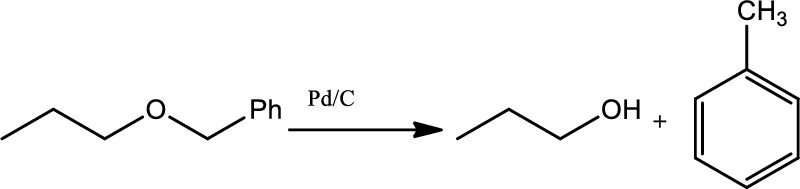
Chapters 22 roadmap reaction legend,
Reaction 22.1:
Halogenation:
In halogenation reaction, aromatic ring reacts with chlorine in the presence of lewis acid catalyst
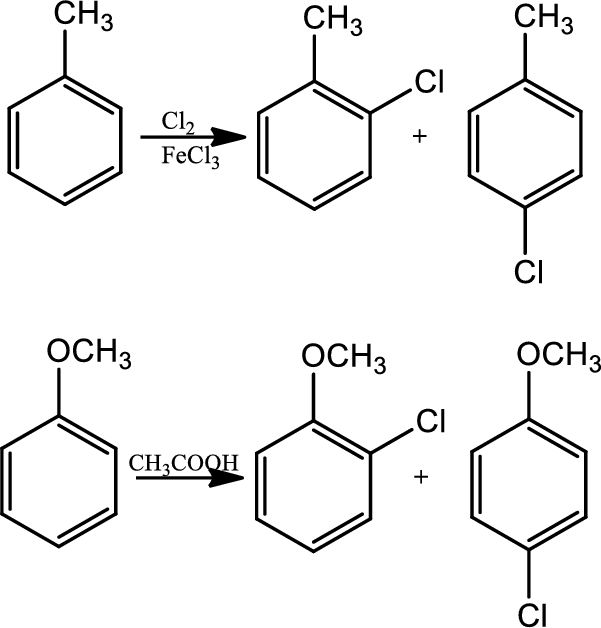
Reaction 22.2:
Nitration:
This is a type of electrophilic substitution on aromatic ring. The electrophile is the nitronium ion
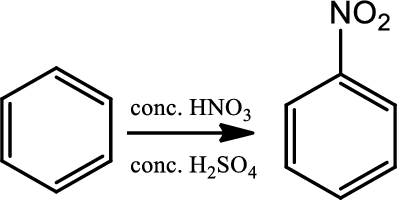
Reaction 22.3:
Sulfonation:
This is a type of electrophilic substitution on aromatic ring. The electrophile is either
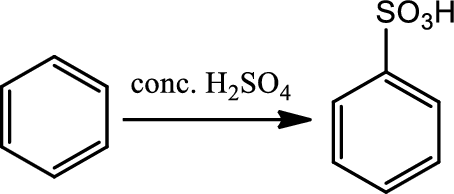
Reaction 22.4:
Fridel-craft’s alkylation:
This is a type of electrophilic substitution on aromatic ring. The electrophile is a carbocation formed as an ion pair interaction of haloalkane with lewis acid. Rearrangements from a less stable carbocation to more stable carbocation are common, the mechanism involves an initial reaction between the haloalkane and lewis acid
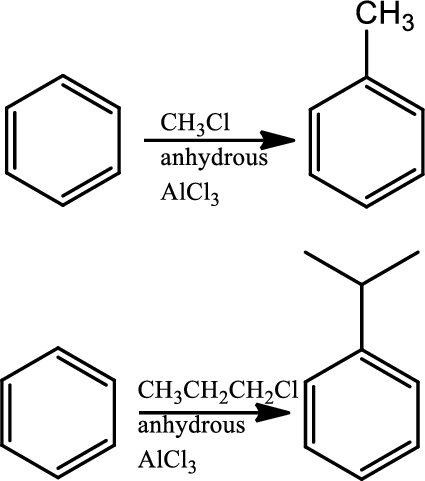
Reaction 22.5:
Fridel-craft’s acylation:
This is a type of electrophilic substitution on aromatic ring. The electrophile is an acylium cation formed as an ion pair by interaction of an acyl halide with a lewis acid. The mechanism involves an initial reaction between the acid chloride and lewis acid
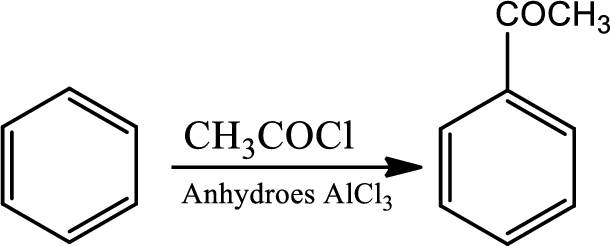
Reaction 22.6:
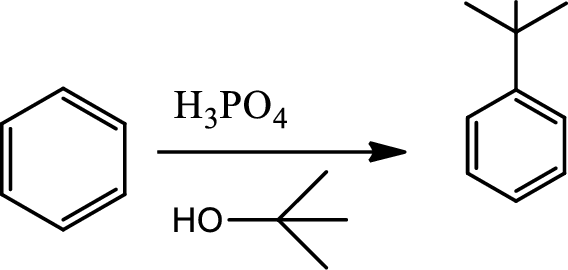
Alkylation using alkene:
This is a type of electrophilic substitution on aromatic ring. The electrophile is a carbocation fromed by the reaction between the alkene and acids.
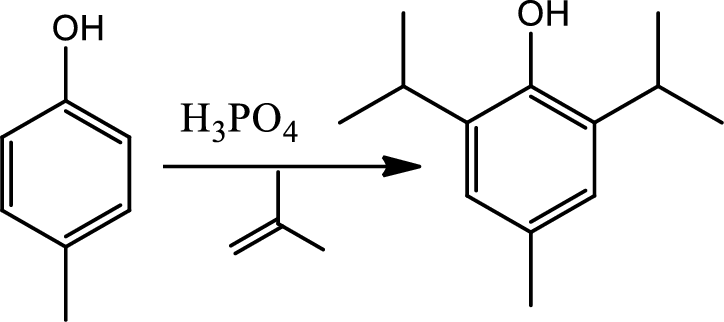
Reaction 22.7:
Alkylation using alcohols:
This is a type of electrophilic substitution on aromatic ring. The electrophile is a carbocation formed from an alcohol reacting with acid.
Chapters 23 roadmap reaction legend,
Reaction 23.1:
Alkylation of ammonia and amine:
Alkylation of amine is a type of organic reaction between an alkyl halide and ammonia or amine. This is nucleophilic aliphatic substitution and the reaction product is higher substitued amine. But the problem with the reaction is the overalkylation.
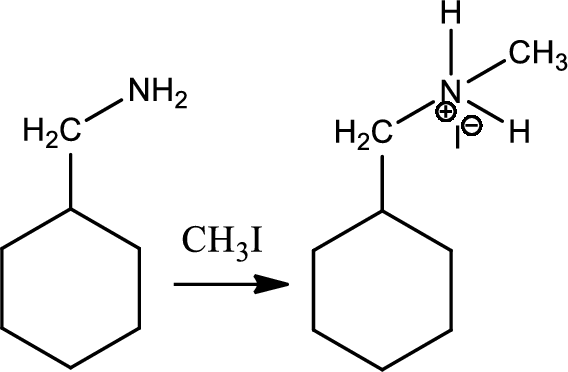
Reaction 23.2:
Alkylation of azide ion followed by reduction:
Azides are prepared by the treatment of a primary or secondary haloakane or an epoxide with
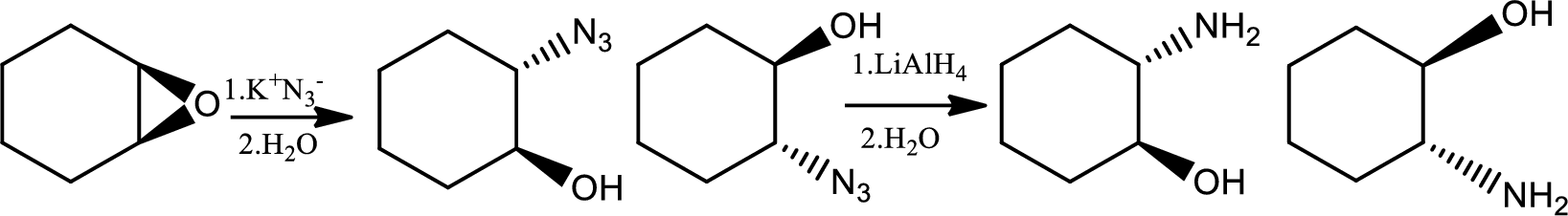
Reaction 23.3:
Nitrosation of tertiary aromatic amines:
This is an electrophilic aromatic substitution. The electrophile here is the nitrosyl cation which is very weak and participates in electrophilic aromatic substitution only with highly activated aromatic rings.
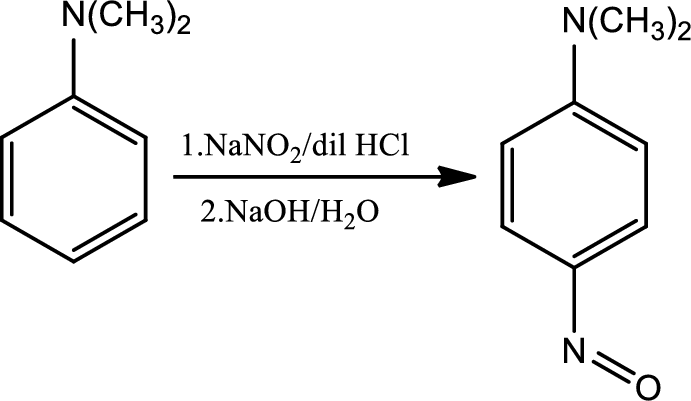
Reaction 23.4:
Formation of N-nitrosamine from secondary amines:
This is an electrophilic aromatic substitution. The electrophile here is the nitrosyl cation which reacts with secondary amine to give N-nitrsoamine.
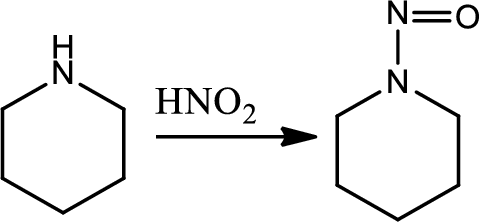
Reaction 23.5:
Diazotisation reaction:
Diazonium salta are organic compounds having
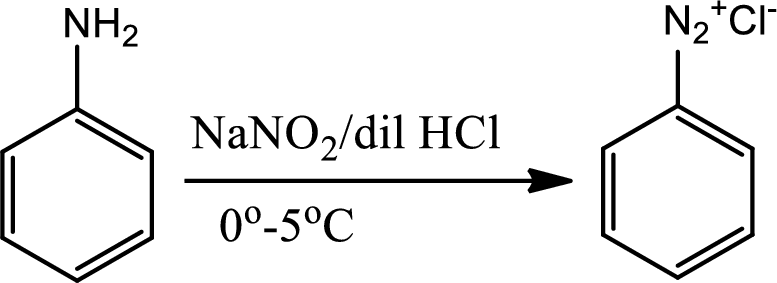
Reaction 23.6:
Conversion of primary aryl amine to phenol:
Formation of an arenediazonium salt followed by loss of nitrogen gives an aryl cation intermediate which then reacts with water to give a phenol.
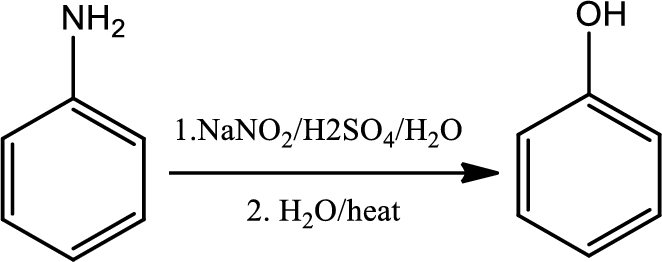
Reaction 23.7:
Schiemann reaction:
The Schiemann reaction is a chemical reaction in which a primary amine is transformed to an aryl fluoride via diazonium tetrafluoroborate intermediate.
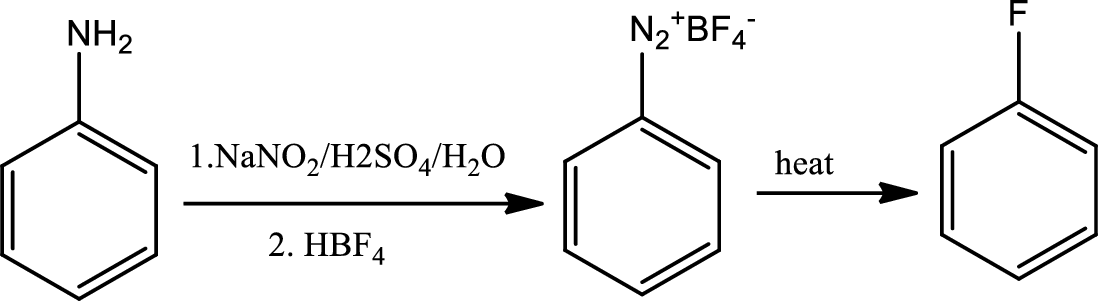
Reaction 23.8:
Sandmeyer reaction:
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which the unique transformation on benzene such as halogenation, cyanation, trifluoromethylation and hydroxylations can be done.
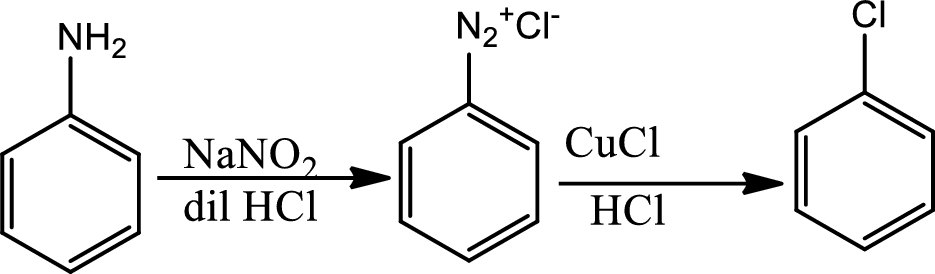
Reaction 23.9:
Reaction of arenediazonium salt with KI:
Reaction of arenediazonium salt with KI is the most convinient way of introducing iodide to benzene ring.
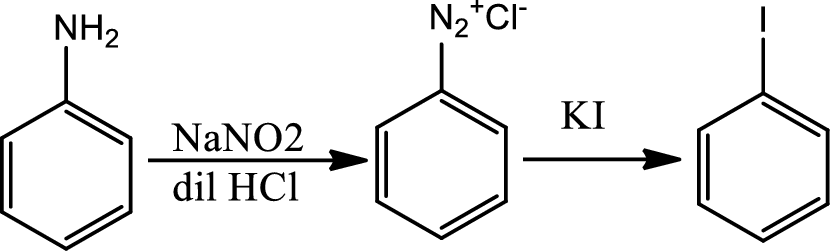
Reaction 23.10:
Reduction of arenediazonium salt with hypophosphorus acid:
By this pathway amine or nitro group can be removed from benzene ring.
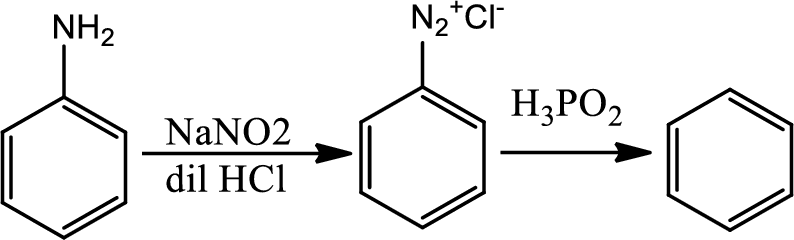
Reaction 23.11:
Hoffmann elimination:
Reaction of a quarternary ammonium halide with moist silver oxide to produce a quarternary ammonium hydroxide followed by heating to give an alkene is a reaction known as Hoffmann elimination. The mechanism involves the simultenous deprotonation of a beta hydrogen by base and los of the amino group in an anti geometry. In this process less substituted alkene is formed.
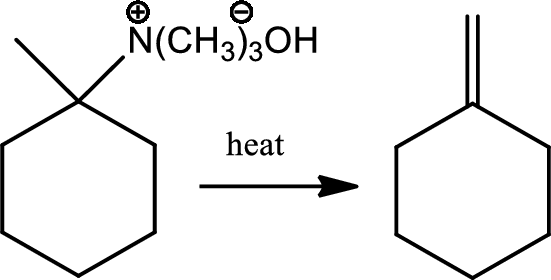
Reaction 23.12:
Cope elimination:
Treatment of the tertiary amine with hydrogen peroxide gives an amine oxide which on heating gives an alkene and an N,N-dialkylhydroxyamine in the reaction is known as Cope elimination. The elimination is syn stereoselective and involves a cyclic flow of six electrons in planar transition state.
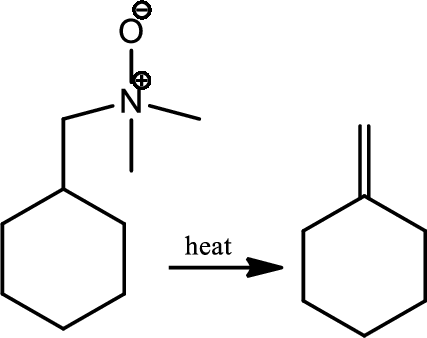
Reaction 23.12:
Reaction of cyclic beta amino alcohol with nitrous acid:
Treating a cyclic beta amino alcohol with nitrous acid leads to rearrangement and a ring expanded ketone. The mechanism involves an initial formation of diazonium ion followed by simulteneous loss of nitrogen and rearrangement by a 1,2-shift to give a resonance stabilised cation that loses a proton to give the product cyclic ketone.
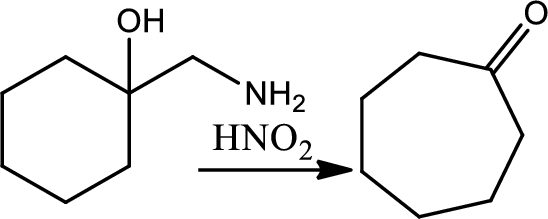
Thus, the reaction roadmap for the reactions in the study Guide section of chapters can be made as shown below for the chapters question 20-23,
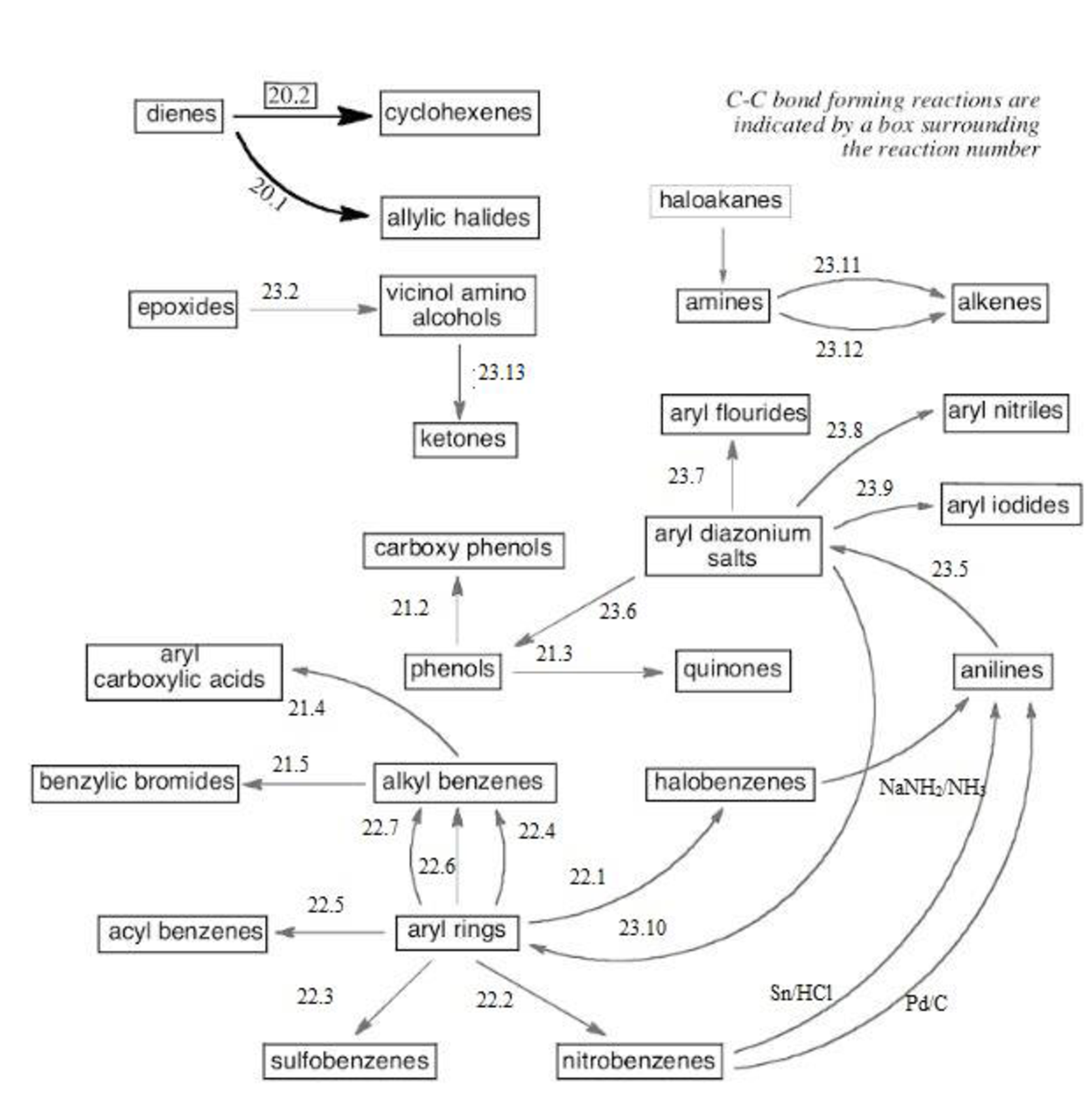
Figure-1
Want to see more full solutions like this?
Chapter 20 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- The data for the potential difference of a battery and its temperature are given in the table. Calculate the entropy change in J mol-1 K-1 (indicate the formulas used).Data: F = 96485 C mol-1arrow_forwardIn a cell, the change in entropy (AS) can be calculated from the slope of the E° vs 1/T graph. The slope is equal to -AS/R, where R is the gas constant. Is this correct?arrow_forwardUsing the Arrhenius equation, it is possible to establish the relationship between the rate constant (k) of a chemical reaction and the temperature (T), in Kelvin (K), the universal gas constant (R), the pre-exponential factor (A) and the activation energy (Ea). This equation is widely applied in studies of chemical kinetics, and is also widely used to determine the activation energy of reactions. In this context, the following graph shows the variation of the rate constant with the inverse of the absolute temperature, for a given chemical reaction that obeys the Arrhenius equation. Based on the analysis of this graph and the concepts acquired about the kinetics of chemical reactions, analyze the following statements: I. The activation energy (Ea) varies with the temperature of the system. II. The activation energy (Ea) varies with the concentration of the reactants. III. The rate constant (K) varies proportionally with temperature. IV. The value of the…arrow_forward
- In an electrolytic cell, indicate the formula that relates E0 to the temperature T.arrow_forward-- 14:33 A Candidate Identification docs.google.com 11. Compound A can transform into compound B through an organic reaction. From the structures below, mark the correct one: HO A تھے۔ די HO B ○ A) Compounds A and B are isomers. B) Both have the same number of chiral carbons. C) Compound A underwent an addition reaction of Cl2 and H2O to form compound B. D) Compound A underwent a substitution reaction forming the intermediate chlorohydrin to obtain compound B. E) Compound A underwent an addition reaction of Cl2 forming the chloronium ion and then added methanol to obtain compound B. 60arrow_forward-- 14:40 A Candidate Identification docs.google.com 13. The compound 1-bromo-hex-2-ene reacts with methanol to form two products. About this reaction, mark the correct statement: OCH3 CH3OH Br OCH3 + + HBr A B A) The two products formed will have the same percentage of formation. B) Product B will be formed by SN1 substitution reaction with the formation of an allylic carbocation. C) Product A will be formed by SN1 substitution reaction with the formation of a more stable carbocation than product B. D) Product A will be formed by an SN2 substitution reaction occurring in two stages, the first with slow kinetics and the second with fast kinetics. E) The two compounds were obtained by addition reaction, with compound B having the highest percentage of formation. 57arrow_forward
- -- ☑ 14:30 A Candidate Identification docs.google.com 10. Amoxicillin (figure X) is one of the most widely used antibiotics in the penicillin family. The discovery and synthesis of these antibiotics in the 20th century made the treatment of infections that were previously fatal routine. About amoxicillin, mark the correct one: HO NH2 H S -N. HO Figura X. Amoxicilina A) It has the organic functions amide, ester, phenol and amine. B) It has four chiral carbons and 8 stereoisomers. C) The substitution of the aromatic ring is of the ortho-meta type. D) If amoxicillin reacts with an alcohol it can form an ester. E) The structure has two tertiary amides. 62arrow_forwardThe environmental police of a Brazilian state received a report of contamination of a river by inorganic arsenic, due to the excessive use of pesticides on a plantation on the riverbanks. Arsenic (As) is extremely toxic in its many forms and oxidation states. In nature, especially in groundwater, it is found in the form of arsenate (AsO ₄ ³ ⁻ ), which can be electrochemically reduced to As ⁰ and collected at the cathode of a coulometric cell. In this case, Potentiostatic Coulometry (at 25°C) was performed in an alkaline medium (pH = 7.5 throughout the analysis) to quantify the species. What potential (E) should have been selected/applied to perform the analysis, considering that this is an exhaustive electrolysis technique (until 99.99% of all AsO ₄ ³ ⁻ has been reduced to As ⁰ at the electrode, or n( final) = 0.01% n( initial )) and that the concentration of AsO ₄ ³ ⁻ found in the initial sample was 0.15 mmol/L ? Data: AsO ₄ 3 ⁻ (aq) + 2 H ₂ O ( l ) + 2 e ⁻ → A s O ₂ ⁻ ( a…arrow_forward-- 14:17 15. Water-soluble proteins are denatured when there is a change in the pH of the environment in which they are found. This occurs due to the protonation and deprotonation of functional groups present in their structure. Choose the option that indicates the chemical bonds modified by pH in the protein represented in the following figure. E CH2 C-OH CH2 H₂C H₁C CH CH3 CH3 CH CH₂-S-S-CH₂- 910 H B -CH2-CH2-CH2-CH₂-NH3* −0—C—CH₂- ○ A) A, C e D. • В) Вес ○ C) DeE ○ D) B, De E ○ E) A, B e C 68arrow_forward
- Suppose sodium sulfate has been gradually added to 100 mL of a solution containing calcium ions and strontium ions, both at 0.15 mol/L. Indicate the alternative that presents the percentage of strontium ions that will have precipitated when the calcium sulfate begins to precipitate. Data: Kps of calcium sulfate: 2.4x10 ⁻ ⁵; Kps of strontium sulfate: 3.2x10 ⁻ ⁷ A) 20,2 % B) 36,6 % C) 62,9 % D) 87,5 % E) 98.7%arrow_forward14:43 A Candidate Identification docs.google.com 14. The following diagrams represent hypothetical membrane structures with their components numbered from 1 to 6. Based on the figures and your knowledge of biological membranes, select the correct alternative. | 3 5 || 人 2 500000 6 A) Structures 1, 3, 5, 2 and 4 are present in a constantly fluid arrangement that allows the selectivity of the movement ○ of molecules. Structure 4, present integrally or peripherally, is responsible for this selection, while the quantity of 6 regulates the fluidity. B) The membranes isolate the cell from the environment, but allow the passage of water-soluble molecules thanks to the presence of 2 and 3. The membrane in scheme is more fluid than that in 55arrow_forward12. Mark the correct statement about reactions a and b : a. Br + -OH Br b. + Br H₂O + Br -OH + H₂O A) The reactions are elimination reactions, with reaction "a" being of type E2 and reaction "b" being of type E1. B) Reaction "a" is an E2 type elimination occurring in one step and reaction "b" is an SN1 type substitution. C) Both reactions can result in the formation of carbocation, but in reaction "b" the most stable carbocation will be formed. D) Both reactions occur at the same rate ○ and have the same number of reaction steps. E) Reaction "b" is an E2 type elimination occurring in two steps and reaction "a" is an SN2 type substitution.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
