
Point i in Fig. 20-19 represents the initial state of an ideal gas at temperature T. Taking algebraic signs into account, rank the entropy changes that the gas undergoes as it moves, successively and reversibly, from point i to points a, b, c, and d, greatest first.
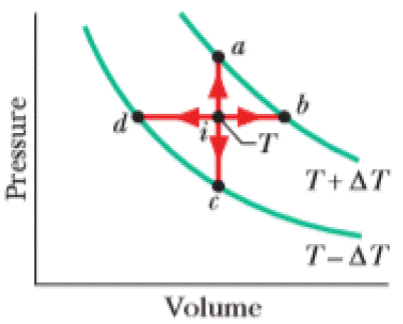
Figure 20-19 Question 1.
To rank:
The entropy changes that the gas undergoes as it moves successively and reversibly from point
Answer to Problem 1Q
Solution:
The ranking of the change of entropy of the gas is
Explanation of Solution
1) Concept:
We can compare the entropy changes of the gas at different points from specific heat and temperature at that points using the relation between change in entropy, specific heat, and temperature at the given point.
2) Formulae:
i)
ii)
3) Given:
The figure showing point
4) Calculations:
In
There are four processes in which two of them are at a higher temperature and two of them are at a lower temperature. The points
The process in which heat is absorbed leads to an increase in the temperature and entropy of the gas. S, o the change of entropy of the gas is positive.
The process that releases energy in the form of heat leads to decrease in entropy. i.e.
The molar specific heat at constant pressure is greater than constant volume, i.e.
The points
For an isobaric process,
For an isochoric process,
So the change of entropy is larger for the isobaric process.
Hence, entropy change is greater at point b and d than at point a and c.
Since b is at a higher temperature than that of d and a is at a higher temperature than that of c.
Therefore, the ranking of the entropy changes of the gas is
Conclusion:
Entropy change depends on temperature and specific heat of an ideal gas.
Want to see more full solutions like this?
Chapter 20 Solutions
Fundamentals Of Physics 11th Edition Loose-leaf Print Companion Volume 2 With Wileyplus Card Set
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Human Anatomy & Physiology (2nd Edition)
Chemistry: Structure and Properties (2nd Edition)
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
Organic Chemistry (8th Edition)
- 20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?arrow_forwardpls help on allarrow_forwardpls help on thesearrow_forward
- pls help on all asked questions kindlyarrow_forwardpls help on all asked questions kindlyarrow_forward19. Mount Everest, Earth's highest mountain above sea level, has a peak of 8849 m above sea level. Assume that sea level defines the height of Earth's surface. (re = 6.38 × 106 m, ME = 5.98 × 1024 kg, G = 6.67 × 10 -11 Nm²/kg²) a. Calculate the strength of Earth's gravitational field at a point at the peak of Mount Everest. b. What is the ratio of the strength of Earth's gravitational field at a point 644416m below the surface of the Earth to a point at the top of Mount Everest? C. A tourist watching the sunrise on top of Mount Everest observes a satellite orbiting Earth at an altitude 3580 km above his position. Determine the speed of the satellite.arrow_forward
- pls help on allarrow_forwardpls help on allarrow_forward6. As the distance between two charges decreases, the magnitude of the electric potential energy of the two-charge system: a) Always increases b) Always decreases c) Increases if the charges have the same sign, decreases if they have the opposite signs d) Increases if the charges have the opposite sign, decreases if they have the same sign 7. To analyze the motion of an elastic collision between two charged particles we use conservation of & a) Energy, Velocity b) Momentum, Force c) Mass, Momentum d) Energy, Momentum e) Kinetic Energy, Potential Energyarrow_forward

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





