
Point i in Fig. 20-19 represents the initial state of an ideal gas at temperature T. Taking algebraic signs into account, rank the entropy changes that the gas undergoes as it moves, successively and reversibly, from point i to points a, b, c, and d, greatest first.
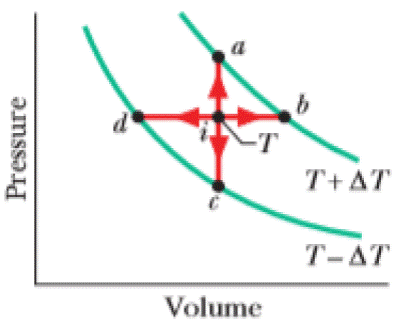
Figure 20-19 Question 1.
To rank:
The entropy changes that the gas undergoes as it moves successively and reversibly from point
Answer to Problem 1Q
Solution:
The ranking of the change of entropy of the gas is
Explanation of Solution
1) Concept:
We can compare the entropy changes of the gas at different points from specific heat and temperature at that points using the relation between change in entropy, specific heat, and temperature at the given point.
2) Formulae:
i)
ii)
3) Given:
The figure showing point
4) Calculations:
In
There are four processes in which two of them are at a higher temperature and two of them are at a lower temperature. The points
The process in which heat is absorbed leads to an increase in the temperature and entropy of the gas. S, o the change of entropy of the gas is positive.
The process that releases energy in the form of heat leads to decrease in entropy. i.e.
The molar specific heat at constant pressure is greater than constant volume, i.e.
The points
For an isobaric process,
For an isochoric process,
So the change of entropy is larger for the isobaric process.
Hence, entropy change is greater at point b and d than at point a and c.
Since b is at a higher temperature than that of d and a is at a higher temperature than that of c.
Therefore, the ranking of the entropy changes of the gas is
Conclusion:
Entropy change depends on temperature and specific heat of an ideal gas.
Want to see more full solutions like this?
Chapter 20 Solutions
Fundamentals of Physics Extended 10e Binder Ready Version + WileyPLUS Registration Card
Additional Science Textbook Solutions
Biology: Life on Earth (11th Edition)
Human Anatomy & Physiology (2nd Edition)
Chemistry: Structure and Properties (2nd Edition)
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
Organic Chemistry (8th Edition)
- Mick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?arrow_forwardHi, I have canceled, why did you charge me again?arrow_forwardNo chatgpt pls will upvotearrow_forward

 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





