
Concept explainers
One structure for the acetoxonium ion is
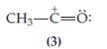
Clearly, the receptor is the positively charged
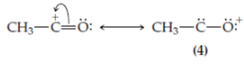
structure (4) is obtained in which the oxygen possesses a formal positive charge but, in addition, both the oxygen and the carbon atom lack a stable octet. This structure will be very unstable. Structure (4), despite the fact that it can be generated by properly pushing electrons, is not included in the resonance hybrid for the acetoxonium ion. If, on the other hand, the unshared electrons on the oxygen are pushed, a more acceptable structure is obtained, namely,
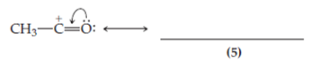
In structure (5) the oxygen atom possesses a formal positive charge, and both the carbon and the oxygen atom have a stable octet. Structure (5) is included in the resonance hybrid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
EBK PUSHING ELECTRONS

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

