Chemistry (OER)
2nd Edition
ISBN: 9781947172616
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 30E
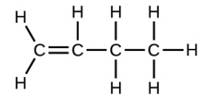
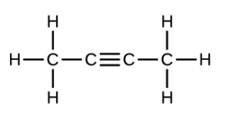
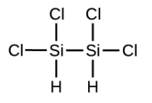
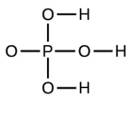
Write the molecular and empirical formulas of the following compounds:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the IUPAC name of the following compound?
CH₂CH₂
H
CI
H₂CH₂C
H
CH₂
Selected Answer:
O
(35,4R)-4 chloro-3-ethylpentane
Correct
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
I
I
I
H
Select to Add Arrows
HCI, CH3CH2OH
Chapter 2 Solutions
Chemistry (OER)
Ch. 2 - In the following drawing, the green spheres...Ch. 2 - Which postulate of Dalton’s theory is consistent...Ch. 2 - Identify the postulate of Dalton’s theory that is...Ch. 2 - Samples of compound X, Y, and Z are analyzed, with...Ch. 2 - The existence of isotopes violates one of the...Ch. 2 - How are electrons and protons similar? How are...Ch. 2 - How are protons and neutrons similar? How are they...Ch. 2 - Predict and test the behavior of a particles fired...Ch. 2 - Predict and test the behavior of a particles fired...Ch. 2 - In what way are isotopes of a given element always...
Ch. 2 - Write the symbol for each of the following ions:...Ch. 2 - Write the symbol for each of the following ions:...Ch. 2 - Open the Build an Atom simulation...Ch. 2 - Open the Build an Atom simulation...Ch. 2 - Open the Build an Atom simulation...Ch. 2 - Determine the number of protons, neutrons, and...Ch. 2 - The following are properties of isotopes of two...Ch. 2 - Give the number of protons, electrons, and...Ch. 2 - Give the number of protons, electrons, and...Ch. 2 - Click on the site...Ch. 2 - Click on the site...Ch. 2 - An element has the following natural abundances...Ch. 2 - Average atomic masses listed by JUPAC are based on...Ch. 2 - Variations in average atomic mass may be observed...Ch. 2 - The average atomic masses of some elements may...Ch. 2 - The 18O:16O abundance ratio in some meteorites is...Ch. 2 - Explain why the symbol for an atom of the element...Ch. 2 - Explain why the symbol for the element sulfur and...Ch. 2 - Write the molecular and empirical formulas of the...Ch. 2 - Write the molecular and empirical formulas of the...Ch. 2 - Determine the empirical formulas for the following...Ch. 2 - Determine the empirical formulas for the following...Ch. 2 - Write the empirical formulas for the following...Ch. 2 - Open the Build a Molecule simulation...Ch. 2 - Open the Build a Molecule simulation...Ch. 2 - Open the Build a Molecule simulation...Ch. 2 - Using the periodic table, classify each of the...Ch. 2 - Using the periodic table, classify each of the...Ch. 2 - Using the periodic table, Identify the lightest...Ch. 2 - Using the periodic table, Identify the heaviest...Ch. 2 - Use the periodic table to give the name and symbol...Ch. 2 - Use the periodic table to give the name and symbol...Ch. 2 - Write a symbol for each of the following neutral...Ch. 2 - Write a symbol for each of the following neutral...Ch. 2 - Using the periodic table, predict whether the...Ch. 2 - Using the periodic table, predict whether the...Ch. 2 - For each of the following compounds, state whether...Ch. 2 - For each of the following compounds, state whether...Ch. 2 - For each of the following pairs of ions, write the...Ch. 2 - For each of the following pairs of ions, write the...Ch. 2 - Name the following compounds: CsCl BaO K2S BeCl2...Ch. 2 - Name the following compounds: NaF Rb2O BCl3 H2Se...Ch. 2 - Write the formulas of the following compounds:...Ch. 2 - Write the formulas of the following compounds:...Ch. 2 - Write the formulas of the following compounds:...Ch. 2 - Write the formulas of the following compounds:...Ch. 2 - Each of the following compounds contains a metal...Ch. 2 - Each of the following compounds contains a metal...Ch. 2 - The following ionic compounds are found in common...Ch. 2 - The following ionic compounds are found in common...Ch. 2 - What are the IUPAC names of the following...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Q1. Carbon tetrachloride has a chlorine - to- carbon mass ratio of 11.8:1. If a simple of carbon tetrachloride ...
Introductory Chemistry (6th Edition)
Body, Heal Thyself The precision of mitotic cell division is essential for repairing damaged tissues like those...
Biology: Life on Earth with Physiology (11th Edition)
Police Captain Jeffers has suffered a myocardial infarction. a. Explain to his (nonmedically oriented) family w...
Human Physiology: An Integrated Approach (8th Edition)
How do you think a cell performing cellular respiration rids itself of the resulting CO2?
Campbell Biology in Focus (2nd Edition)
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
- Explain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forwardDraw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY