
Concept explainers
(a)
Interpretation:
The polarity of the given molecule is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
The given molecule A is nonpolar.
Explanation of Solution
![]()
The given molecule is in trans form. The directions of the vectors of both the C-F bonds are equal but opposite to each other. Hence the dipole moments of both the C-F bonds get cancelled out with each other. Therefore, there is no net dipole moment.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule A is nonpolar.
(b)
Interpretation:
The polarity of the given molecule is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
The given molecule B is polar.
Explanation of Solution
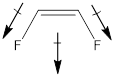
The given molecule is in cis form. The direction of vectors of both the C-F bonds is in the same direction, giving a net permanent dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule B is polar.
(c)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule C is nonpolar.
Explanation of Solution
![]()
In this molecule, both the C-Cl bonds are opposite to each other, so the dipole moments are cancelled out with each other. Therefore, there is no net dipole moment in this molecule.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule C is nonpolar.
(d)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a.
Answer to Problem 2.41P
The given molecule D is polar.
Explanation of Solution
![]()
In this molecule, chlorine is more electronegative than the carbon atom; hence the direction of the vector of dipole moment is more towards C-Cl bond, giving a net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule D is polar.
(e)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule E is polar.
Explanation of Solution
![]()
In this molecule, Chlorine is more electronegative than bromine; hence the direction of the vector of dipole moment is more towards C-Cl bond, giving a net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule E is polar.
(f)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule F is nonpolar.
Explanation of Solution
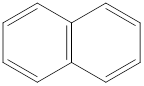
In this molecule, there is no electronegative atom present since no charge separation is taking place. So there is no net dipole moment.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule F is nonpolar.
(g)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule G is polar.
Explanation of Solution
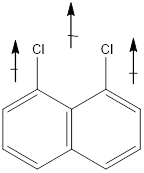
In this molecule, Chlorine is an electronegative atom, and both the C-Cl bonds are in the same direction. Therefore, the direction of the vector of dipole is moment is upward, giving a net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule G is polar.
(h)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule H is nonpolar.
Explanation of Solution
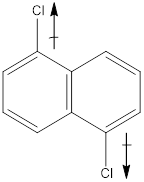
In the molecule, chlorine is an electronegative atom, and both the C-Cl bonds are in opposite direction. Therefore, the directions of the vectors of dipole moment of two C-Cl bonds get cancelled out with each other. Hence there is no net dipole moment.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule H is nonpolar.
(i)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule I is polar.
Explanation of Solution
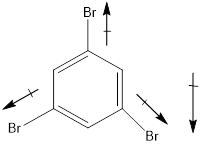
In this molecule, bromine is an electronegative atom, but one C-Br bond is in upward direction, and two C-Br bonds are in downward direction. Therefore, the net dipole moment acts in downward direction.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule I is polar.
(j)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule J is polar.
Explanation of Solution
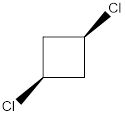
In this molecule, both the C-Cl bonds are present above the plane (that is wedge notation). Therefore, the directions of the vectors of dipole moment of both the C-Cl bonds are in the same direction, giving net dipole moment to the molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule J is polar.
(k)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule K is polar.
Explanation of Solution
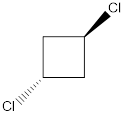
In this molecule, one C-Cl bond is present above the plane (that is, the wedge notation), and another C-Cl bond is present below the plane (that is, the dotted notation). Therefore, the directions of the vectors of dipole moment of both the C-Cl bonds are in opposite direction, which get cancelled out with each other, giving no net dipole moment to the molecule.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule K is nonpolar.
(l)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule L is polar.
Explanation of Solution

In this molecule, though both the C-Cl bonds are in opposite direction, both the chlorines are present on carbon
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule L is polar.
(m)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule M is nonpolar.
Explanation of Solution
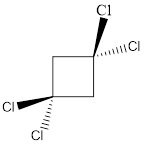
In this molecule, two C-Cl bonds are above the plane, and two C-Cl bonds are below the plane; hence the molecule has symmetry. The directions of the vectors of dipole moment of all the four C-Cl bonds are cancelled with each other, giving no net dipole moment to the molecule.
Dipole moment on this molecule is symmetrically distributed; hence the given molecule M is nonpolar.
(n)
Interpretation:
The polarity of the given molecules is to be determined.
Concept introduction:
The dipole moment of a molecule is a measure of the magnitude of its dipole. A dipole moment is a vector, which has both magnitude and direction. Bond polarity originates from bonds between atoms of different electronegativity. Symmetry of molecules also predicts the polarity of a molecule.
Answer to Problem 2.41P
Molecule N is polar.
Explanation of Solution
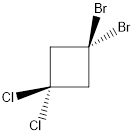
In this molecule, two C-Cl and two C-Br bonds are present. Since chlorine is more electronegative than bromine, the direction of the vector of dipole moment is towards C-Cl bonds. Therefore, there is a net dipole moment present in this molecule.
Dipole moment on this molecule is not symmetrically distributed; hence the given molecule N is polar.
Want to see more full solutions like this?
Chapter 2 Solutions
Get Ready for Organic Chemistry
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
- 个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forward
- DE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



