
EBK INORGANIC CHEMISTRY
5th Edition
ISBN: 9780133558944
Author: Tarr
Publisher: PEARSON CUSTOM PUB.(CONSIGNMENT)
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 2.41P
The second ionization energy of He ¡s almost exactly four times the ionization energy of H,and the third ionization energy of Li is almost exactly nine times the ionization energy of H:
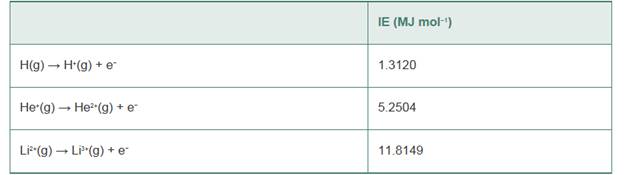
Explain this trend on the basis of the Bohr equation for energy levels of single-electron systems.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When two solutions, one of 0.1 M KCl (I) and the other of 0.1 M MCl (II), are brought into contact by a membrane. The cation M cannot cross the membrane. At equilibrium, x moles of K+ will have passed from solution (I) to (II). To maintain the neutrality of the two solutions, x moles of Cl- will also have to pass from I to II. Explain this equality: (0.1 - x)/x = (0.1 + x)/(0.1 - x)
Calculate the variation in the potential of the Pt/MnO4-, Mn2+ pair with pH, indicating the value of the standard potential. Data: E0 = 1.12.
Given the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt. Calculate the emf of the cell as a function of pH.
Chapter 2 Solutions
EBK INORGANIC CHEMISTRY
Ch. 2.1 - Determine the energy of the transition from nh=3...Ch. 2.2 - Describe the angular nodal surfaces for a dz2...Ch. 2.2 - Prob. 2.3ECh. 2.2 - A third possible state for the p4 configuration...Ch. 2.2 - A nitrogen atom, with three 2p electrons, could...Ch. 2.2 - Calculate the effective nuclear charge on a 5s,...Ch. 2.2 - Calculate the effective nuclear charge on a 7s,...Ch. 2.3 - Explain why all three graphs in Figure 2.14 have...Ch. 2 - Determine the de Brogue wavelength of a. an...Ch. 2 - Using the equation E=RH(1221nh2) determine the...
Ch. 2 - The transition from the n=7 to the n=2 level of...Ch. 2 - Emissions are observed at wavelengths of 383.65...Ch. 2 - What is the least amount of energy that can be...Ch. 2 - Hydrogen atom emission spectra measured from the...Ch. 2 - The Rydberg constant equation has two terms that...Ch. 2 - For the 3pz and 4dxz hydrogen-like atomic...Ch. 2 - Repeat the exercise in Problem 2.7 for the 4s and...Ch. 2 - Repeat the exercise in Problem 2.7 for the 5s and...Ch. 2 - The 4fz(x2y2) orbital has the angular function...Ch. 2 - Prob. 2.13PCh. 2 - The label for an fz2 orbital, like that for a dz2...Ch. 2 - a. Determine the possible values for the l and ml...Ch. 2 - a. What are the values of quantum numbers I and n...Ch. 2 - a. At most, how many electrons in an atom can have...Ch. 2 - Determine the Coulombic and exchange energies for...Ch. 2 - Prob. 2.19PCh. 2 - Prob. 2.20PCh. 2 - What states are possible for a d3 configuration?...Ch. 2 - Provide explanations of the following phenomena:...Ch. 2 - Give electron configurations for the following:...Ch. 2 - Predict the electron configurations of the...Ch. 2 - Radial probability plots shed insight on issues of...Ch. 2 - Briefly explain the following on the basis of...Ch. 2 - Briefly explain the following on the basis of...Ch. 2 - a. Which 2+ ion has two 3d electrons? Which has...Ch. 2 - A sample calculation in this chapter showed that,...Ch. 2 - Ionization energies should depend on the effective...Ch. 2 - Prepare a diagram such as the one in Figure (a)...Ch. 2 - Why are the ionization energies of the alkali...Ch. 2 - The second ionization of carbon (C+C2++e) and the...Ch. 2 - Prob. 2.35PCh. 2 - Prob. 2.36PCh. 2 - The second ionization energy involves removing an...Ch. 2 - Prob. 2.38PCh. 2 - On the basis of electron configurations, explain...Ch. 2 - a. The graph of ionization energy versus atomic...Ch. 2 - The second ionization energy of He ¡s almost...Ch. 2 - The size of the transition-metal atoms decreases...Ch. 2 - Predict the largest and smallest radius in each...Ch. 2 - Select the best choice, and briefly indicate the...Ch. 2 - Select the best choice, and briefly indicate the...Ch. 2 - There are a number of Web sites that display...Ch. 2 - Prob. 2.47P
Additional Science Textbook Solutions
Find more solutions based on key concepts
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
Give the IUPAC name for each compound.
Organic Chemistry
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The decimolar calomel electrode has a potential of 0.3335 V at 25°C compared to the standard hydrogen electrode. If the standard reduction potential of Hg22+ is 0.7973 V and the solubility product of Hg2Cl2 is 1.2x 10-18, find the activity of the chlorine ion at this electrode.Data: R = 8.314 J K-1 mol-1, F = 96485 C mol-1, T = 298.15 K.arrow_forward2. Add the following group of numbers using the correct number of significant figures for the answer. Show work to earn full credit such as rounding off the answer to the correct number of significant figures. Replace the question marks with the calculated answers or write the calculated answers near the question marks. 10916.345 37.40832 5.4043 3.94 + 0.0426 ? (7 significant figures)arrow_forwardThe emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.arrow_forward
- Indicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.arrow_forwardHow can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forward
- Using Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forwardThe molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forward
- BA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY