
a)
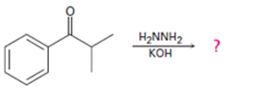
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism, beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
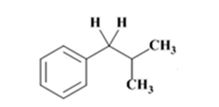
The electron-pushing mechanism for the formation of the alkane, beginning from the hydrazone intermediate, is given below.
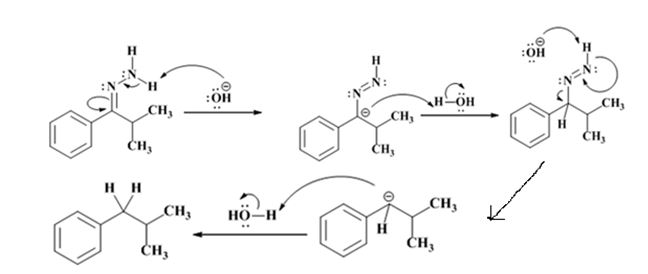
Explanation of Solution
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of isopropyl phenyl ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane, ethyl benzene, as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism, beginning from the hydrazone intermediate is given below.

b)
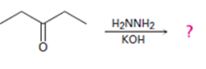
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction aldehydes and ketones react with hydrazine in the presence of a base to yield alkanes. First a hydrazone is formed which is then converted into an alkane.
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism the formation of the alkane beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
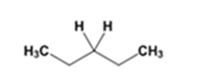
The electron-pushing mechanism the formation of the alkane, beginning from the hydrazone intermediate, is given below.
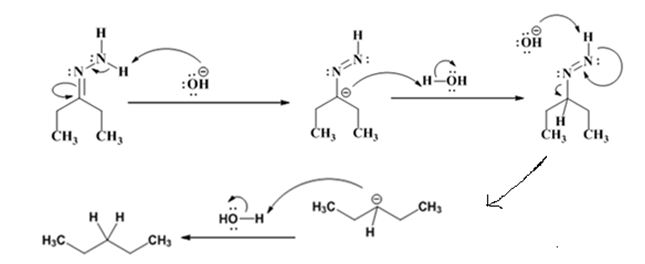
Explanation of Solution
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of diethyl ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane, n-pentane, as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism, beginning from the hydrazone intermediate is given below.

c)
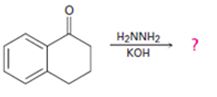
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction aldehydes and ketones react with hydrazine in the presence of a base to yield alkanes. First a hydrazone is formed which is then converted into an alkane.
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism the formation of the alkane beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
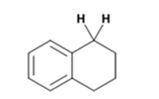
The electron-pushing mechanism the formation of the alkane, beginning from the hydrazone intermediate, is given below.
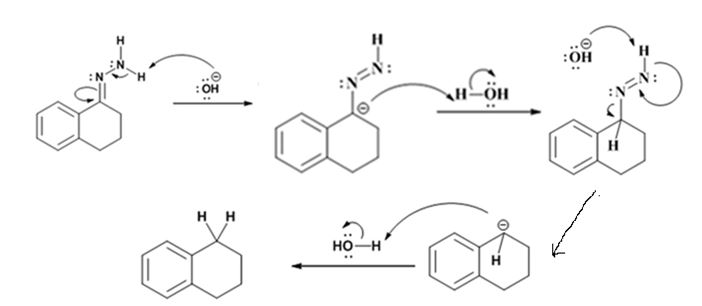
Explanation of Solution
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of the ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism, beginning from the hydrazone intermediate is given below.

d)
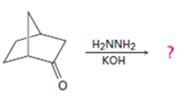
Interpretation:
The product of the Wolff-Kishner reduction reaction shown is to be given. The electron-pushing mechanism, beginning from the hydrazone intermediate is to be provided.
Concept introduction:
In Wolff-Kishner reduction aldehydes and ketones react with hydrazine in the presence of a base to yield alkanes. First a hydrazone is formed which is then converted into an alkane.
To give:
The product of the Wolff-Kishner reduction reaction shown.
To provide:
The electron-pushing mechanism, beginning from the hydrazone intermediate.
Answer to Problem 38MP
The product of the Wolff-Kishner reduction reaction shown is
The electron-pushing mechanism for the formation of the alkane beginning from the hydrazone intermediate, is given below.
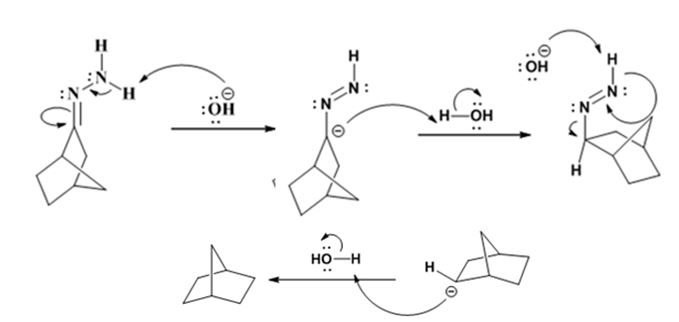
Explanation of Solution
The hydroxide ion from KOH abstracts a weakly acidic H from –NH2 of the hydrazone of ketone to yield a carbanion which picks up a proton to yield a neutral intermediate. Deprotonation of the remaining hydrogen on N by the hydroxide ion occurs with the eliminartion of nitrogen to yield another carbanion which is protonated to give the alkane as the product.
The product of the Wolff-Kishner reduction reaction shown is

The electron-pushing mechanism for the formation of the alkane beginning from the hydrazone intermediate, is given below.

Want to see more full solutions like this?
Chapter 19 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- identify the carbonyl compound that is incapable of forming an enolate ionarrow_forwardpredict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forward
- Is (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward© Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forwardThree pure compounds are formed when 1.00 g samples of element x combine with, respectively, 0.472 g, 0.630 g, and 0.789 g of element z. The first compound has the formula x2Z3. find the empricial formula of the other two compoundsarrow_forward
- Draw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forwardIn reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


