
Concept explainers
Interpretation:
On the basis of the structure, determine whether the given molecule is a
Concept introduction:
Our body produces a number of proteins.
The amino acids in the proteins are coded by
Nucleic acids are
Each nucleotide is made up of base, sugar, and phosphate group.
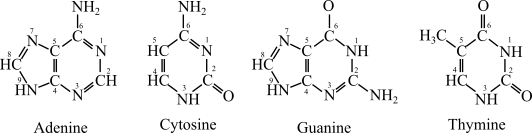
The nucleotides differ in their base unit, which can either be adenine (A), guanine (G), cytosine (C), uracil (U) and thymine (T).
In DNA, the four bases are adenine (A), guanine (G), cytosine (C), and thymine (T).
In RNA uracil replaces thymine, the four bases are adenine (A), guanine (G), cytosine (C), and uracil (U).
The structure of nitrogenous bases is given as below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Introductory Chemistry, Books a la Carte Plus Mastering Chemistry with Pearson eText -- Access Card Package (6th Edition)
- Deducing a rate law from the change in concentration over time To exit full screen, press and hold esc A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) → H₂O(aq) +CO₂ (aq) - She fills a reaction vessel with H2CO3 and measures its concentration as the reaction proceeds: time (milliseconds) [H2CO3] 0 0.0500 M 10. 0.0266M 20. 0.0181 M 30. 0.0138M 40. 0.0111 M Use this data to answer the following questions. Write the rate law for this reaction. Calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. rate ☐ x10 k = Х 000 18 Ararrow_forwardWriting the rate law implied by a simple mechanism Suppose the formation of tert-butanol proceeds by the following mechanism: step elementary reaction 1 (CH3)3 CBr(aq) → (CH3)2 C* (aq) + Br (aq) 2 (CH3)2C (aq) + OH¯ (aq) → (CH3)2COH(aq) rate constant k₁ k₂ Suppose also k₁ »k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. rate = k ☐ Express the rate constant k for the overall chemical reaction in terms of K1, K2, and (if necessary) the rate constants k-1 and K-2 for the reverse of the two elementary reactions in the mechanism. k = ☐ □ ☑ G ? 00. 18 Ar Barrow_forwardDeducing a rate law from the change in concentration over time A chemistry graduate student is studying the rate of this reaction: 2SO3 (g) →>> 2SO2 (g) + O2(g) He fills a reaction vessel with SO3 and measures its concentration as the reaction proceeds: ? time (minutes) [SO3] 0 0.0200M 1.0 0.0105 M 2.0 0.00552M 3.0 0.00290M 4.0 0.00152M Use this data to answer the following questions. Write the rate law for this reaction. rate = k ☐ x10 Calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. k = ☐ Х 000 18 Ar BAarrow_forward
- Using the Arrhenius equation to calculate k at one temperature from k at... The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E reaction is 1.2 × 107 M −1 .S at 160.0 °C, what will the rate constant be at 194.0 °C? Round your answer to 2 significant digits. k = Шм −1 -1 .S ☐ x10 ☑ 5 = = 16.0 kJ/mol. If the rate constant of this a ? olo Ar Barrow_forwardUsing the Arrhenius equation to calculate k at one temperature from k at... a The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E = 10.0 kJ/mol. If the rate constant of this reaction is 9.9 × 107 M -1 .S at 246.0 °C, what will the rate constant be at 196.0 °C? Round your answer to 2 significant digits. k = ☐ M -1 −1 .S x10 ☑ ? 00. 18 Ar Barrow_forwardWriting the rate law implied by a simple mechanism Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: elementary reaction - NO(g) + Br2(g) → NOBг2(g) step 1 2 NOBг2(g) + NO(g) - rate constant k₁ 2 NOBr(g) k2 Suppose also k₁ »k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. ☐ rate = k Express the rate constant k for the overall chemical reaction in terms of k₁, k2, and (if necessary) the rate constants k-1 and K-2 for the reverse of the two elementary reactions in the mechanism. = ☐ ロ→ロ Х ك ? 000 18 Ararrow_forward
- Deducing a rate law from the change in concentration over time chemistry graduate student is studying the rate of this reaction: 2H3PO4 (aq) → P₂O5 (aq) +3H₂O (aq) 2 e fills a reaction vessel with H3PO and measures its concentration as the reaction proceeds: 4 time (seconds) [H3PO4] 0 0.500M 1.0 0.229 M 2.0 0.148M 3.0 0.110M 4.0 0.0871 M se this data to answer the following questions. Write the rate law for this reaction. rate = k x10 Calculate the value of the rate constant k. k = Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. ☑ G olo 18 Ararrow_forwardWriting the rate law implied by a simple mechanism Suppose the formation of nitrosyl chloride proceeds by the following mechanism: elementary reaction step rate constant 1 NO(g) + Cl2(g) → NOC₁₂(g) k₁ 2 NOCl2(g) + NO(g) 2 NOCl(g) →>> k2 Suppose also k₁ »k. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. rate = k ☐ Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of K1, K2, and (if necessary) the rate constants k-1 and K-2 for the reverse of the two elementary reactions in the mechanism. k = | Х ? 18 Ararrow_forwardUsing first- and second-order integrated rate laws 1/5 Consider this reaction: H2CO3(aq) → H₂O (aq) +CO₂ (aq) At a certain temperature it obeys this rate law. rate = (2.27 s¹) [H2CO3] Suppose a vessel contains H2CO3 at a concentration of 0.830M. Calculate how long it takes for the concentration of H2CO3 to decrease by 83.0%. You may assume no other reaction is important. Round your answer to 2 significant digits. S x10 ☑ § ? 00. 18 Ararrow_forward
- Using the Arrhenius equation to calculate k at one temperature from k at... Try Again Your answer is incorrect. 0/5 a The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E = 28.0 kJ/mol. If the rate constant of this -1 -1 reaction is 2.5 × 10³ M ·S at 45.0 °C, what will the rate constant be at 104.0 °C? Round your answer to 2 significant digits. ST -1 -1 ☐ x10 k = 2.8 × 10 - M .S 18 Ararrow_forwardIn the theory of the state of transition, indicate the expression of the constant k in function of deltaE0#. This expression is also the ecuation of Arrhenius?arrow_forwardBriefly indicate the differences between Ea of the theory of collisions and E0# the theory of the state of transition.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





