
Concept explainers
(a)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The
Answer to Problem 43E
The class of compound
Explanation of Solution
The given organic compound is
Amines are the derivatives of ammonia. The formula of ammonia is
When the hydrogen of the ammonia is replaced by alkyl group amines is formed. The general formula of amines is shown below.
Therefore, the class of compound
The class of given has been rightfully identified.
(b)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of compound
Explanation of Solution
The given organic compound is
Halogen is the elements that belong to the seventeenth group of the periodic table. When a halogen is combined with a hydrocarbon, then organic halides are formed. Fluorine is halogen.
Therefore, the class of compound
The class of given has been rightfully identified.
(c)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is ester.
Explanation of Solution
The structure of the given compounds is shown below.
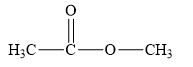
Figure 1
Esters are the derivatives of carboxylic acid. The general structure of carboxylic acid is shown below.
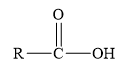
Figure 2
When the hydrogen of the hydroxyl group of carboxylic acid is replaced by another alkyl group, then ester is formed.
Therefore, the class of the given compound is ester.
The class of given has been rightfully identified.
(d)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is a carboxylic acid.
Explanation of Solution
The structure of the given organic compound is shown below.
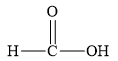
Figure 3
The given compound can release
The given compound is an organic acid.
Therefore, the class of the given compound is carboxylic acid.
The class of given has been rightfully identified.
(e)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is aldehyde.
Explanation of Solution
The structure of the given organic compound is shown below.
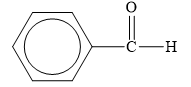
Figure 4
The carbonyl compounds are the compounds that contain
Therefore, the class of the given compound is aldehyde.
The class of given has been rightfully identified.
(f)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of given compound is ether.
Explanation of Solution
The structure of the given organic compound is shown below.
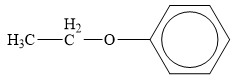
Figure 5
Ether is the derivatives of alcohol. The general formula of alcohols is shown below.
When the hydrogen of the alcohol is replaced by another alkyl group ether is formed.
Therefore, the class of given compound is ether.
The class of given has been rightfully identified.
(g)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of given compound is ketone.
Explanation of Solution
The structure of the given organic compound is shown below.
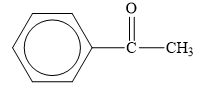
Figure 6
The carbonyl compounds are the compounds that contain
Therefore, the class of the given compound is ketone.
The class of given has been rightfully identified.
(h)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is amide.
Explanation of Solution
The structure of the given compounds is shown below.
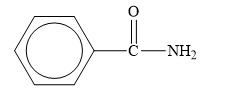
Figure 7
Amides are the derivatives of carboxylic acid. The general structure of carboxylic acid is shown below.
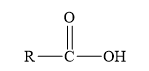
Figure 2
When the hydroxyl group of a carboxylic acid is replaced by amine group, then amide is formed.
Therefore, the class of the given compound is amide.
The class of given has been rightfully identified.
Want to see more full solutions like this?
Chapter 19 Solutions
EBK INTRODUCTORY CHEMISTRY
- Michael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forwardRank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forward
- The following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forward
- Which one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





