
In Figure P19.22, the change in internal energy of a gas that is taken from A to C along the blue path is +800 J. The work done on the gas along the red path ABC is −500 J. (a) How much energy must be added to the system by heat as it goes from A through B to C? (b) If the pressure at point A is five times that of point C, what is the work done on the system in going from C to D?
Figure P19.22
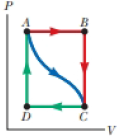
(c) What is the energy exchanged with the surroundings by heat as the gas goes from C to A along the green path? (d) If the change in internal energy in going from point D to point A is +500 J, how much energy must be added to the system by heat as it goes from point C to point D?
(a)
Answer to Problem 22P
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation of conservation of energy.
Here,
Substitute
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from A through B to C is
(b)
Answer to Problem 22P
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation to calculate the work done on the system from C to D.
Here,
Here,
The pressure at point A is five times that of point C.
And the volume is,
Substitute
Substitute
Conclusion:
Therefore, the work done on the system from C to D if pressure at A is five times that of point C is
(c)
Answer to Problem 22P
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Write the equation to calculate work done along the green path from C to A.
Here, the volume
Substitute 0 for
Substitute
Substitute
Conclusion:
Therefore, the energy exchanged with the surroundings by heat along the green path from C to A is
(d)
Answer to Problem 22P
is
Explanation of Solution
Given info: The change in internal energy of a gas which is taken along the blue path from A to C is
Write the equation of first law of thermodynamics.
Here,
Substitute
Conclusion:
Therefore, the energy which must be added to the system by heat which goes from C to D is
Want to see more full solutions like this?
Chapter 19 Solutions
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
- 8. With the aid of a diagram draw the following electric circuit and use the resistor as the load, (a) Closed circuit (b) Open circuitarrow_forwardLab 8 Part 3 PHET Wave Interface simulation. I am having trouble with this part of the lab.arrow_forwardMick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





