
To find:
a) Sketch the titration curve for the titration of
b) Label the curve with the
c) Draw the structures of the species present in the solution at the equivalence point.
Answer to Problem 19.77QA
Solution:
a) and b) Labeled titration curve for the titration of
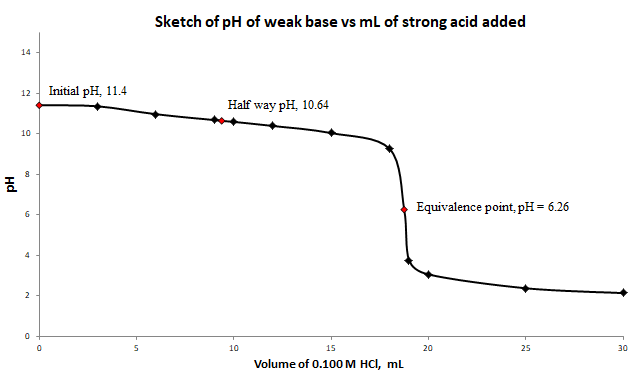
c) At the equivalence point, the structure of the species present in the solution is
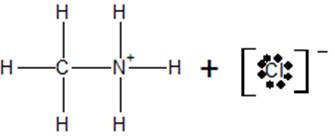
Explanation of Solution
1) Concept:
We can calculate moles of weak base present and the moles of
We know how much of the weak base remains and how much of its conjugate acid produced because one mole of base reacts with one mole of
2) Formula:
i)
ii)
iii)
iv)
v)
vi)
vii)
3) Given:
i)
ii) Volume of methylamine =
iii)
iv) For methylamine,
v) For methylamine,
4) Calculation:
a) Calculation of initial
| Reaction | |||
| Initial | |||
| Change | |||
| Final | |||
Applying law of mass action,
Solving this equation using quadratic equation formula:
The negative value of concentration has no significance. Therefore, we will proceed with
So,
Therefore, initial
At half way to the equivalence point,
Therefore, at half way to the equivalence point,
At equivalence point, the moles of base is the same as moles of acid.
Calculation of moles of weak base methylamine:
Calculation of moles of
Calculation of volume of
| Reaction | |||
| Initial | |||
| Change | |||
| Final | |||
Therefore, at equivalence point, only
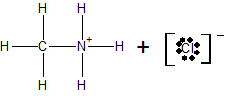
Calculation of total volume of solution at equivalence point:
Total volume =
Calculation of concentration of
| Reaction | |||
| Initial | |||
| Change | |||
| Final | |||
Calculation of
Applying the law of mass action:
We assume that the value of
Therefore,
At equivalence point, the
b) Label the curve with the

c) At the equivalence point, the structure of the species present in the solution is
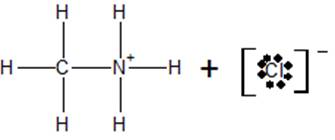
Conclusion:
The initial
Want to see more full solutions like this?
Chapter 19 Solutions
Chemistry: An Atoms-Focused Approach
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





