
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 40P
Give the IUPAC name for each compound.
a. 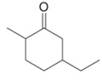 c.
c. 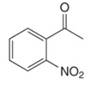 e.
e. 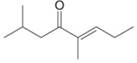
b. 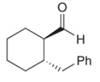 d.
d. 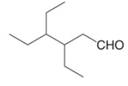 f.
f. 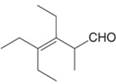
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2.
Add the following group of numbers using the correct number of significant figures for the
answer. Show work to earn full credit such as rounding off the answer to the correct number
of significant figures. Replace the question marks with the calculated answers or write
the calculated answers near the question marks.
10916.345
37.40832
5.4043
3.94
+
0.0426
?
(7 significant figures)
The emf at 25°C of the cell: Pt l H2(g) l dis X:KCl (sat) l Hg2Cl2(s) l Hg l Pt was 612 mV. When solution X was replaced by normal phosphate buffer solution with a pH of 6.86, the emf was 741 mV. Calculate the pH of solution X.
Indicate how to calculate the potential E of the reaction Hg2Cl2(s) + 2e ⇄ 2Hg + 2Cl- as a function of the concentration of Cl- ions. Data: the solubility product of Hg2Cl2.
Chapter 18 Solutions
ORGANIC CHEMISTRY
Ch. 18.1 - Rank the following compounds in order of...Ch. 18.1 - Prob. 2PCh. 18.2 - Give the IUPAC name for each aldehyde.Ch. 18.2 - Prob. 4PCh. 18.2 - Give the IUPAC name for each ketone.Ch. 18.5 - Prob. 11PCh. 18.9 - Problem 21.17 Draw the products of the following...Ch. 18.9 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 18.9 - Problem 21.19 Draw the products (including...Ch. 18.9 - Problem 21.20 What starting materials are needed...
Ch. 18.9 - Prob. 19PCh. 18.10 - Problem 21.22 The product formed when reacts with...Ch. 18.10 - Prob. 21PCh. 18.11 - Prob. 22PCh. 18.11 - Prob. 23PCh. 18.11 - Prob. 24PCh. 18.12 - Prob. 25PCh. 18.12 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 18.13 - Problem 21.29 Draw the products of each...Ch. 18 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 18 - 21.41 Rank the following compounds in order of...Ch. 18 - Prob. 39PCh. 18 - 21.43 Give the IUPAC name for each compound.
a....Ch. 18 - 21.44 Give the structure corresponding to each...Ch. 18 - Prob. 42PCh. 18 - 21.46 Draw the products of each reaction.
a. e....Ch. 18 - Prob. 44PCh. 18 - 21.48 Draw all stereoisomers formed in each...Ch. 18 - Prob. 54PCh. 18 - Prob. 55PCh. 18 - Prob. 56PCh. 18 - Devise a synthesis of each alkene using a Wittig...Ch. 18 - Prob. 60PCh. 18 - Prob. 62PCh. 18 - Prob. 63PCh. 18 - 21.64 Draw a stepwise mechanism for the following...Ch. 18 - 21.65 Draw a stepwise mechanism f or the following...Ch. 18 - Prob. 67PCh. 18 - 21.67 Draw a stepwise mechanism for each...Ch. 18 - Prob. 69PCh. 18 - Prob. 70P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How can Beer’s Law be used to determine the concentration in a selected food sample. Provide an in-depth discussion and examples of this.arrow_forwardb) H3C- H3C Me CH 3 I HN Me H+arrow_forwardUsing Luther's rule, determine the reference potentials of the electrodes corresponding to the low stability systems Co³+/Co and Cr²+/Cr from the data in the table. Electrodo ΕΝ Co²+/Co Co3+/Co²+ -0,28 +1,808 Cr³+ / Cr -0,508 Cr3+ / Cr²+ -0,41arrow_forward
- The molecule PYRIDINE, 6tt electrons and is there pore aromuntre and is Assigned the Following structure contenus Since aromatk moleculey undergo electrophilic allomatic substitution, Pyridine should undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this roaction Based upon the reaction the reaction mechanism determine which of these producty would be the major Product of the hegetionarrow_forwardUsing Benzene as starting materia Show how each of the Following molecules could Ve synthesked 9. CHI d. 10450 b 0 -50311 ८ City -5034 1-0-650 e NO2arrow_forwardBA HBr of the fol 1)=MgCI 2) H₂O major NaOEt Ts Cl Py (pyridine) 1) 03 2) Me2S 1arrow_forward
- 4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a) NHBoc ⚫OBn HO. H3C CO2CH3 -OBn H3C H3C. H3C. NHBOC CI CO2CH3arrow_forwardDraw structures of the following compounds and identify their role: mCPBA (MCPBA) DMS Py 9-BBN LAH Sia₂BH TsCI PCC t-BuOK LDA MeLi n-BuLi DMSO DMF Sodium Borohydride Lithium DiisopropylAmide 2arrow_forwardUsing Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode. DATA: Electrode potentials E° = 0,854 V y E 0,788 V Hg2+/Hg 2+ Hg2/Hgarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY