
Concept explainers
(a) Give the IUPAC name for A and B. (b) Draw the product formed when A or B is treated with each reagent: 1.
(a)
Interpretation: The IUPAC name for A and B is to be determined.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the carbon chain containing higher number of carbon atoms.
2. For functional group such as aldehyde suffix ‘al’ and for ketone suffix ‘one’ is added to the name.
3. When the chain is substituted with different substituents, then the numbering is done according to priority.
Answer to Problem 37P
The IUPAC name for A and B is
Explanation of Solution
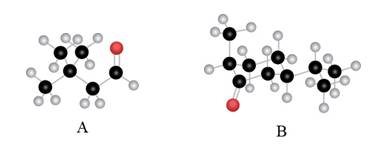
The given compound is,
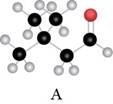
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
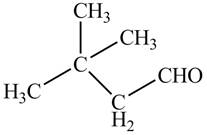
Figure 2
The parent hydrocarbon is butane. The functional group present is aldehyde. Two methyl groups are present on third carbon atom. When same substituents are present, then prefix depends on the number of substituents. The IUPAC name of the compound is
The given compound is,
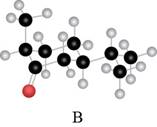
Figure 3
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
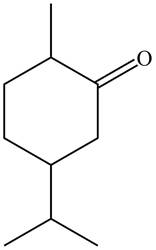
Figure 4
The parent hydrocarbon is cyclohexane. The functional group present is ketone. Methyl group is present on fifth carbon atom and isopropyl group is present on third carbon atom. The IUPAC name of the compound is
The IUPAC name for A and B is
(b)
Interpretation: The products formed when B is treated with given reagents are to be drawn.
Concept introduction: The metal hydride reagents are good reducing agents such as
Grignard reagents are organometallic compounds which are prepared using alkyl halides in presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases.
Answer to Problem 37P
The products formed when B is treated with given reagents are,
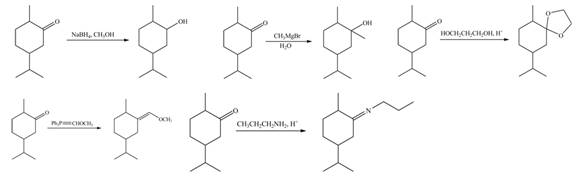
Explanation of Solution
1. In the presence of
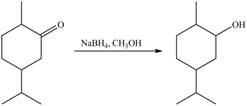
Figure 5
The product formed is
2. The Grignard reagent reacts with compound B to form secondary alcohol. The corresponding reaction is shown below.
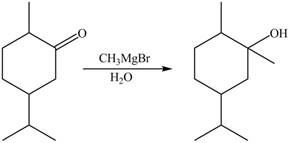
Figure 6
The product formed is
3. When compound B is treated with
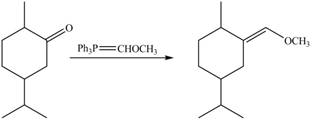
Figure 7
The product formed is
4. The compound B reacts with
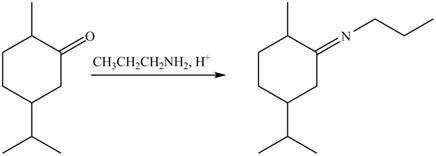
Figure 8
The product formed is
5. The product formed by the reaction of compound B with ethylene glycol in the presence of
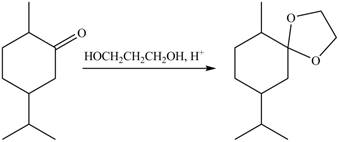
Figure 9
The products formed when B is treated with given reagents are shown in Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9.
Want to see more full solutions like this?
Chapter 18 Solutions
EBK ORGANIC CHEMISTRY
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
- In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forwardCalculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forward
- If the normal potential for the Fe(III)/Fe(II) pair in acid at zero pH is 524 mV Hg/Hg2Cl2 . The potential of the saturated calomel reference electrode is +246 mV versus the NHE. Calculate E0 vs NHE.arrow_forwardGiven the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.arrow_forwardIndicate the functions that salt bridges have in batteries.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
