
Concept explainers
The synthesis of cyclobutanecarboxylic acid given in Section 18.7 was first carried out by William Perkin, Jr., in 1883, and it represent-ed one of the first syntheses of an organic compound with a ring smaller than six carbon atoms. (There was a general feeling at the time that such compounds would be too unstable to exist.) Earlier in 1883, Perkin reported what he mistakenly believed to be a cyclobutane derivative obtained from the reaction of acetoacetic ester and 1,3-dibromopropane. The reaction that Perkin had expected to take place was the following:
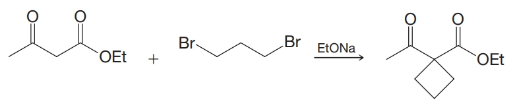
The molecular formula for his product agreed with the formulation given in the preceding reaction, and alkaline hydrolysis and acidification gave a nicely crystalline acid (also having the expected molecular formula). The acid, however, was quite stable to heat and resisted decarboxylation. Perkin later found that both the ester and the acid contained six-membered rings (five carbon atoms and one oxygen atom). Recall the charge distribution in the enolate ion obtained from acetoacetic ester and propose structures for Perkin’s ester and acid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
ORGANIC CHEMISTRY-WILEYPLUS ACCESS PKG.
Additional Science Textbook Solutions
Living By Chemistry: First Edition Textbook
Organic Chemistry (8th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Anatomy & Physiology (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology (11th Edition)
- State the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.arrow_forwardWhy are normal electrode potentials also called relative electrode potentials?arrow_forwardEasily differentiate between electrochemical potential and Galvani potential.arrow_forward
- Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forwardplease help with hwarrow_forwardhelp me solve this hwarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

