
Interpretation: The correct coefficients for each reactant and product are to be chosen for the following reaction from the given options:
a) 2, 1, 4 → 2, 1, 2
b) 1, 1, 2 → 1, 1, 1
c) 2, 1, 2 → 2, 1, 1
d) 2, 1, 2 → 2, 1, 2
Answer to Problem 1SAQ
Solution:
To solve this problem, begin by assigning oxidation states to all atoms and identify the substances being oxidized and reduced.
Explanation of Solution
The equation for this reaction as follows:
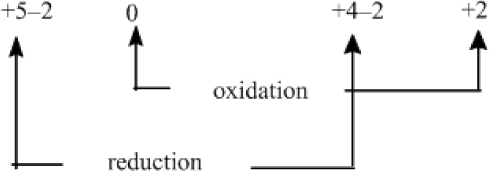
Separate the overall reaction into two half-reactions: one for oxidation and other for reduction.
Balance each half-reaction; balance O by adding
Balance each-half reaction with respect to charge by adding electrons. Make the sum of the charges on both sides of the equation equal by adding as many electrons as necessary.
Make the number of electrons in both-half reactions equal by multiplying one or both half-reactions by a small whole number.
Add the two half-reaction together, cancelling electrons and other species as necessary.
Conclusion:
Thus, the coefficient of each reactant and product will be
Want to see more full solutions like this?
Chapter 18 Solutions
Chemistry: A Molecular Approach & Student Solutions Manual for Chemistry: A Molecular Approach, Books a la Carte Edition Package
- Done 19:17 www-awu.aleks.com Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimited .III LTE סוי 27 28 = 29 30 31 32 = 33 34 35 Consider this structure. CH3CH2CH2 Part 1 of 3 3 CH2 CH2CH3 - C-CH2CH 3 H CH₂ Give the IUPAC name of this structure. 3-ethyl-3,4-dimethylheptane Part: 1/3 Part 2 of 3 Draw the skeletal structure. Skip Part < Check Click and drag to start drawing a structure. Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Хarrow_forward18:57 .III LTE www-awu.aleks.com Chapter 12 HW Question 31 of 39 (8 points) | Question Attem... Give the IUPAC name of each compound. Part 1 of 4 Part 2 of 4 Х Х Check Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. TOMS OF US vacy Center | Accessibilityarrow_forwardWhat is the missing reactant in this organic reaction? CH3-C-CH2-NH2 + R - CH3 O: 0 CH3-N-CH2-C-NH-CH2-C-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click anywhere to draw the first atom of your structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forward
- Done 18:17 • www-awu.aleks.com Chapter 12 HW Question 24 of 39 (4 points) | Question Attempt: 1 of Unlimited ▼ 20 ✓ 21 × 22 23 24 25 26 raw the structure corresponding to each IUPAC name. Part 1 of 2 .III LTE 22 27 28 סוי 29 29 3 A skeletal structure corresponding to the IUPAC name 3-ethyl-4-methylhexane. Part 2 of 2 Click and drag to start drawing a structure. A condensed structure corresponding to the IUPAC name 2,2,4- trimethylpentane. Click anywhere to draw the first atom of your structure. Check Save For Later Submit < Х ப: G © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility : Garrow_forwardDone 18:25 www-awu.aleks.com .III LTE Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimi... Oli 23 24 25 26 27 28 29 30 Consider this structure. CH2 CH2CH2 CH2CH2CH₂ C -C. -CH2CH3 H CH Part: 0 / 3 Part 1 of 3 Give the IUPAC name of this structure. Skip Part < Check ☑ Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ....................arrow_forwardCalculate Ecell at 25.0 oC using the following line notation. Zn(s)|Zn+2(aq, 0.900 M)||Cu+2(aq, 0.000200 M)|Cu(s)arrow_forward
- Predict the product of this organic reaction: O OH + H + OH A P + H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click and drag to start drawing a structure. X G ☐ :arrow_forward0.0994 g of oxalic acid dihydrate is titrated with 10.2 mL of potassium permanganate. Calculate the potassium permanganate concentration. Group of answer choices 0.0433 M 0.135 M 0.0309 M 0.193 Marrow_forwardExperts...can any one help me solve these problems?arrow_forward
- According to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardWhich Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forwardWhich of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





