
General, Organic, and Biochemistry
10th Edition
ISBN: 9781260506198
Author: Denniston, Katherine
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18, Problem 18.75QP
Interpretation Introduction
Interpretation:
The high affinity of carbon monoxide to bind with the heme group reflects its toxicity has to be explained.
Concept Introduction:
The heme group consists of four pyrroles rings attached to iron metal. The four pyrroles rings together are called tetrapyrrole. These tetrapyrrole attached to the side chain helps heme group to hold a metal ion. Together this structure is known as porphyrin ring.
The structure of the heme prosthetic group is,
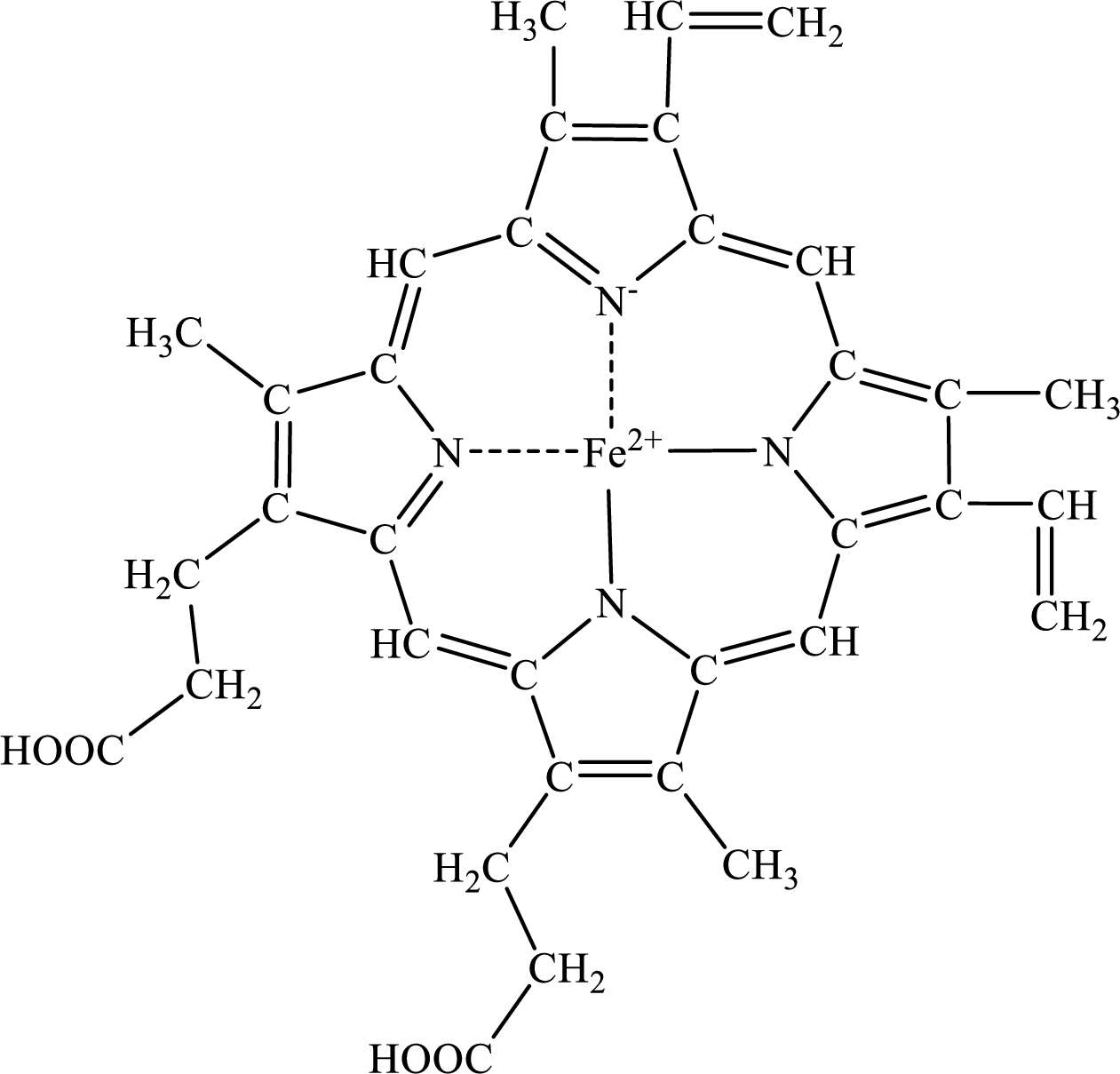
This porphyrin ring can bind to the one molecule of oxygen.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
I
I
I
H
Select to Add Arrows
HCI, CH3CH2OH
Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).
Chapter 18 Solutions
General, Organic, and Biochemistry
Ch. 18.2 - Write the one-letter and three-letter...Ch. 18.2 - Prob. 18.2QCh. 18.3 - Write the structure of each of the following...Ch. 18.3 - Write the structure of each of the following...Ch. 18.3 - Write the structure of each of the following...Ch. 18.7 - Describe the four levels of protein structure.
Ch. 18.7 - Prob. 18.6QCh. 18.9 - Prob. 18.7QCh. 18.9 - Prob. 18.8QCh. 18.11 - Prob. 18.9Q
Ch. 18.11 - How does extremely low pH cause proteins to...Ch. 18.12 - Prob. 18.11QCh. 18.12 - Prob. 18.12QCh. 18 - Prob. 18.13QPCh. 18 - Prob. 18.14QPCh. 18 - Prob. 18.15QPCh. 18 - Prob. 18.16QPCh. 18 - Prob. 18.17QPCh. 18 - Prob. 18.18QPCh. 18 - Prob. 18.19QPCh. 18 - Prob. 18.20QPCh. 18 - Prob. 18.21QPCh. 18 - Prob. 18.22QPCh. 18 - Describe the basic general structure of an...Ch. 18 - Prob. 18.24QPCh. 18 - Prob. 18.25QPCh. 18 - Prob. 18.26QPCh. 18 - Prob. 18.27QPCh. 18 - Prob. 18.28QPCh. 18 - Prob. 18.29QPCh. 18 - Prob. 18.30QPCh. 18 - Prob. 18.31QPCh. 18 - Prob. 18.33QPCh. 18 - Prob. 18.63QPCh. 18 - Prob. 18.64QPCh. 18 - Prob. 18.65QPCh. 18 - Prob. 18.66QPCh. 18 - Prob. 18.67QPCh. 18 - Prob. 18.68QPCh. 18 - Prob. 18.69QPCh. 18 - Prob. 18.70QPCh. 18 - Prob. 18.71QPCh. 18 - Prob. 18.72QPCh. 18 - Prob. 18.73QPCh. 18 - Prob. 18.74QPCh. 18 - Carbon monoxide binds tightly to the heme groups...Ch. 18 - Prob. 18.76QPCh. 18 - Prob. 18.77QPCh. 18 - Prob. 18.78QPCh. 18 - Prob. 18.79QPCh. 18 - Prob. 18.80QPCh. 18 - Prob. 18.81QPCh. 18 - Prob. 18.82QPCh. 18 - Prob. 18.85QPCh. 18 - Prob. 18.86QPCh. 18 - Prob. 18.87QPCh. 18 - Prob. 18.88QPCh. 18 - Prob. 18.89QPCh. 18 - Prob. 18.90QPCh. 18 - Prob. 18.91QPCh. 18 - Prob. 18.92QPCh. 18 - Prob. 18.93QPCh. 18 - Prob. 18.94QPCh. 18 - Prob. 18.95QPCh. 18 - Prob. 18.96QPCh. 18 - Why is it necessary to mix vegetable proteins to...Ch. 18 - Prob. 18.98QPCh. 18 - Prob. 18.99QPCh. 18 - Prob. 18.100QPCh. 18 - Prob. 1MCPCh. 18 - Consider the Chapter Map, and explain the...Ch. 18 - Tardigrades, also known as water bears, are...Ch. 18 - Calculate the length of an α-helical polypeptide...Ch. 18 - Proteins involved in transport of molecules or...Ch. 18 - The α-keratin of hair is rich in the amino acid...Ch. 18 - Calculate the number of different pentapeptides...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forward
- Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- Concentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forwardExplain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forward
- Draw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forwardDraw the condensed structural formula and line-angle formula for each: 2,3-dimethylheptane 3-bromo-2-pentanol 3-isopropyl-2-hexene 4-chlorobutanoic acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY