
Concept explainers
A galvanic cell is constructed as follows. One half-cell consists of a platinum wire immersed in a solution containing 1.0 M Sn2+ and 1.0 M Sn4+; the other half-cell has a thallium rod immersed in a solution of 1.0 M Tl+. (a) Write the half-cell reactions and the overall reaction. (b) What is the equilibrium constant at 25°C? (c) What is the cell voltage if the T1+ concentration is increased tenfold?
(a)
Interpretation:
The half-cell reactions and the overall reactions in the given cell has to be calculated.
Concept Introduction:
Nernst equation is one of the important equation in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
n is the number of electrons involved in a reaction
At room temperature
The relation between electrode potential and equilibrium constant: cell potential and equilibrium constant are related by the given equation.
Where,
The standard electrode potential of a cell
Answer to Problem 18.109QP
The half-cell reactions are,
The overall reaction,
Explanation of Solution
To find the completely balanced chemical equation
The overall cell reaction can be written as given below,
Overall reaction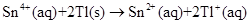
(b)
Interpretation:
The equilibrium constant for the reaction has to be calculated in accordance with the given conditions.
Concept Introduction:
Nernst equation is one of the important equation in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
At room temperature
The relation between electrode potential and equilibrium constant: cell potential and equilibrium constant are related by the given equation.
Where,
The standard electrode potential of a cell
Answer to Problem 18.109QP
The equilibrium constant at
Explanation of Solution
To find the
The standard cell potential can be calculated as given below
To calculate the equilibrium constant.
The equilibrium constant and the electrode potential is related by the given equation.
On rearranging we get,
Hence,
(c)
Interpretation:
The cell potential has to be calculated in accordance with the given conditions.
Concept Introduction:
Nernst equation is one of the important equation in electrochemistry. In Nernst equation the electrode potential of a cell reaction is related to the standard electrode potential, concentration or activities of the species that is involved in the chemical reaction and temperature.
Where,
F isthe Faraday constant
At room temperature
The relation between electrode potential and equilibrium constant: cell potential and equilibrium constant are related by the given equation.
Where,
The standard electrode potential of a cell
Answer to Problem 18.109QP
The cell potential, when the concentration of
Explanation of Solution
To record the given data
The concentration of
The concentration of
The concentration of
Temperature
To find the cell voltage when the concentration
The
Want to see more full solutions like this?
Chapter 18 Solutions
CHEMISTRY-ALEKS 360 ACCESS
- Briefly explain chemical potential.arrow_forwardReason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





