
EBK ESSENTIAL UNIVERSITY PHYSICS, VOLUM
4th Edition
ISBN: 9780135272992
Author: Wolfson
Publisher: VST
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 16E
An ideal gas expands from the state (p1, V1) to the state (p2, V2), where p2 = 2p1 and V2 = 2V1. The expansion proceeds along the diagonal path AB in Fig. 18.19. Find an expression for the work done by the gas during this process.
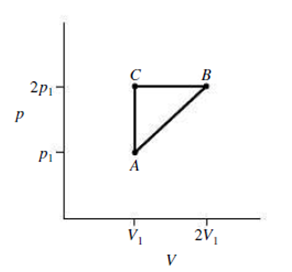
FIGURE 18.19 Exercises 20, 21 and Problem 75
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Lab 8 Part 3 PHET Wave Interface simulation.
I am having trouble with this part of the lab.
Mick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?
Hi,
I have canceled, why did you charge me again?
Chapter 18 Solutions
EBK ESSENTIAL UNIVERSITY PHYSICS, VOLUM
Ch. 18.2 - Two identical gas-cylinder systems are taken from...Ch. 18.2 - Name the basic thermodynamic process involved when...Ch. 18.3 - The same amount of heat flows into equal volumes...Ch. 18 - Prob. 1FTDCh. 18 - Prob. 2FTDCh. 18 - Why cant an irreversible process be described by a...Ch. 18 - Are the initial and final equilibrium states of an...Ch. 18 - Prob. 5FTDCh. 18 - Figure 18.18 shows two processes, A and B. that...Ch. 18 - When you let air out of a tire, the air seems...
Ch. 18 - Blow on the back of your hand with your mouth wide...Ch. 18 - Three identical gas-cylinder systems are...Ch. 18 - Prob. 10FTDCh. 18 - Prob. 11ECh. 18 - Prob. 12ECh. 18 - A 40-W heat source is applied to a gas sample for...Ch. 18 - Find the rate of heat flow into a system whose...Ch. 18 - In a certain automobile engine, 17% of the total...Ch. 18 - An ideal gas expands from the state (p1, V1) to...Ch. 18 - Repeat Exercise 20 for a process that follows the...Ch. 18 - A balloon contains 0.30 mol of helium. It rises,...Ch. 18 - The balloon of Exercise 22 starts at 100 kPa...Ch. 18 - How much work does it take to compress 2.5 mol of...Ch. 18 - By what factor must the volume of a gas with =...Ch. 18 - Prob. 22ECh. 18 - A carbon-sequestration scheme calls for...Ch. 18 - A gas mixture contains 2.5 mol of O2 and 3.0 mol...Ch. 18 - A mixture of monatomic and diatomic gases has...Ch. 18 - What should be the approximate specific-heat ratio...Ch. 18 - Prob. 27ECh. 18 - Prob. 28ECh. 18 - Example 18.2: A gas bubble develops from...Ch. 18 - Prob. 30ECh. 18 - Example 18.2: A spherical balloon is placed inside...Ch. 18 - Prob. 32ECh. 18 - Example 18.4: An ideal gas with γ = 40 and...Ch. 18 - Prob. 34ECh. 18 - Example 18.4: An ideal gas with γ = 1.40 is...Ch. 18 - An ideal gas expands to 10 times its original...Ch. 18 - During cycling, the human body typically releases...Ch. 18 - A 0.25-mol sample of ideal gas initially occupies...Ch. 18 - As the heart beats, blood pressure in an artery...Ch. 18 - It takes 1.5 kJ to compress a gas isothermally to...Ch. 18 - A gas undergoes an adiabatic compression during...Ch. 18 - A gas with = 1.40 occupies 6.25 L when its at...Ch. 18 - A gas sample undergoes the cyclic process ABCA...Ch. 18 - Prob. 44PCh. 18 - A gasoline engine has compression ratio 8.5 (sec...Ch. 18 - By what factor must the volume of a gas with =...Ch. 18 - Volvos B5340 engine, used in the V70 series cars,...Ch. 18 - A research balloon is prepared for launch by...Ch. 18 - Prob. 49PCh. 18 - By what factor does the internal energy of an...Ch. 18 - A 3.50-mol sample of ideal gas with molar specific...Ch. 18 - Prove that the slope of an adiabat at a given...Ch. 18 - An ideal gas with = 1.67 starts at point A in...Ch. 18 - The gas of Example 18.4 starts at state A in Fig....Ch. 18 - The gas of Example 18.4 starts at state A in Fig....Ch. 18 - Prob. 56PCh. 18 - Youre the product safety officer for a company...Ch. 18 - Figure 18.22 shows data and a fit curve from an...Ch. 18 - Gasoline and diesel engines often use...Ch. 18 - A gas with = 7/5 is at 273 K when its compressed...Ch. 18 - An ideal gas with = 1.3 is initially at 273 K and...Ch. 18 - The curved path in Fig. 18.23 lies on the 350-K...Ch. 18 - Repeat part (a) of Problem 62 for the path ACDA in...Ch. 18 - A gas mixture contains monatomic argon and...Ch. 18 - How much of a triatomic gas with Cv = 3R would you...Ch. 18 - An 8.5-kg rock at 0C is dropped into a...Ch. 18 - A piston-cylinder arrangement containing 0.30 mol...Ch. 18 - Experimental studies show that the pV curve for a...Ch. 18 - Show that the application of Equation 18.3 to an...Ch. 18 - Prob. 70PCh. 18 - Prob. 71PCh. 18 - The table below shows measured values of pressure...Ch. 18 - Air with initial volume V0 = 4.50 L and initial...Ch. 18 - A real gas is more accurately described using the...Ch. 18 - Repeat Exercise 20 for an expansion along the path...Ch. 18 - The adiabatic lapse rate is the rate at which air...Ch. 18 - A power plant extracts thermal energy from its...Ch. 18 - Prob. 78PCh. 18 - One scheme for reducing greenhouse-gas emissions...Ch. 18 - Warm winds called Chinooks (a Native-American term...Ch. 18 - Warm winds called Chinooks (a Native-American term...Ch. 18 - Warm winds called Chinooks (a Native-American term...Ch. 18 - Warm winds called Chinooks (a Native-American term...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why are the top predators in food chains most severely affected by pesticides such as DDT?
Campbell Essential Biology (7th Edition)
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Explain all answers clearly, with complete sentences and proper essay structure, if needed. An asterisk (*) des...
Cosmic Perspective Fundamentals
With what geologic feature are the earthquakes in the mid-Atlantic associated?
Applications and Investigations in Earth Science (9th Edition)
A wild-type fruit fly (heterozygous for gray body color and normal wings) is mated with a black fly with vestig...
Campbell Biology (11th Edition)
89. Determine the volume of 0.150 M NaOH solution required to neutralize each sample of hydrochloric acid. The ...
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- You are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?arrow_forwardFor each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank youarrow_forwardA planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).arrow_forward
- What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forward
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY