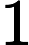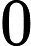CHEMISTRY:CENTRAL SCIENCE-W/MOD.ACCESS
14th Edition
ISBN: 9780134809694
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18, Problem 14E
Step 1:
Interpretation Introduction
To determine: The partial pressure of carbon dioxide when the total atmospheric pressure is .
Step 2:
Interpretation Introduction
To determine: The partial pressure of argon when the total atmospheric pressure is .
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
k
In Davies' equation log y₁ = log-
log y₁ = log | = -Az² + ✓//
k
A is a constant that always equals 1.02. Correct?
- 0,31
Indicate whether the equality is true Yi
How is it obtained?
k%
Indicate the relationship between the activity coefficient YA and the
rate constant of a bimolecular reaction in solution k and the rate
constant at infinite dilution ko.
Chapter 18 Solutions
CHEMISTRY:CENTRAL SCIENCE-W/MOD.ACCESS
Ch. 18.1 - Prob. 18.1.1PECh. 18.1 - Prob. 18.1.2PECh. 18.1 - Prob. 18.2.1PECh. 18.1 - Practice Exercise 2 The bond energy in N2 is 941...Ch. 18.2 - Prob. 18.3.1PECh. 18.2 - Prob. 18.3.2PECh. 18 - Prob. 1DECh. 18 - Prob. 1ECh. 18 - Prob. 2ECh. 18 - The figure shows the three lowest regions of...
Ch. 18 - Prob. 4ECh. 18 - Where does the energy come from to evaporate the...Ch. 18 - Prob. 6ECh. 18 - Prob. 7ECh. 18 - The first stage of treatment at the reverse...Ch. 18 - Prob. 9ECh. 18 - Prob. 10ECh. 18 - Prob. 11ECh. 18 - How are the boundaries between the regions of the...Ch. 18 - Air pollution in the Mexico City metropolitan area...Ch. 18 - Prob. 14ECh. 18 - Prob. 15ECh. 18 - Prob. 16ECh. 18 - Prob. 17ECh. 18 - Prob. 18ECh. 18 - Distinguish between photodissociation and...Ch. 18 - Prob. 20ECh. 18 - Prob. 21ECh. 18 - Prob. 22ECh. 18 - Do the reactions involved in ozone depletion...Ch. 18 - Prob. 24ECh. 18 - Prob. 25ECh. 18 - Prob. 26ECh. 18 - Prob. 27ECh. 18 - Prob. 28ECh. 18 - Prob. 29ECh. 18 - Prob. 30ECh. 18 - Prob. 31ECh. 18 - Prob. 32ECh. 18 - Alcohol-based fuels for automobiles lead to the...Ch. 18 - Prob. 34ECh. 18 - Prob. 35ECh. 18 - Prob. 36ECh. 18 - Prob. 37ECh. 18 - Prob. 38ECh. 18 - Prob. 39ECh. 18 - Prob. 40ECh. 18 - Prob. 41ECh. 18 - Prob. 42ECh. 18 - Although there are many ions in seawater, the...Ch. 18 - The Ogallala aquifer described in the Close Look...Ch. 18 - Prob. 45ECh. 18 - Prob. 46ECh. 18 - List the common products formed when an organic...Ch. 18 - Prob. 48ECh. 18 - Prob. 49ECh. 18 - Prob. 50ECh. 18 - Prob. 51ECh. 18 - Prob. 52ECh. 18 - Prob. 53ECh. 18 - Prob. 54ECh. 18 - Prob. 55ECh. 18 - Prob. 56ECh. 18 - Prob. 57ECh. 18 - Prob. 58ECh. 18 - Prob. 59ECh. 18 - Prob. 60ECh. 18 - Prob. 61AECh. 18 - Prob. 62AECh. 18 - Prob. 63AECh. 18 - Prob. 64AECh. 18 - Prob. 65AECh. 18 - Prob. 66AECh. 18 - Prob. 67AECh. 18 - Explain, using Le Châtelier’s principle, why the...Ch. 18 - Prob. 69AECh. 18 - Prob. 70AECh. 18 - Prob. 71AECh. 18 - Prob. 72AECh. 18 - Prob. 73AECh. 18 - Prob. 74AECh. 18 - Prob. 75AECh. 18 - Prob. 76AECh. 18 - Prob. 77AECh. 18 - Prob. 78IECh. 18 - Prob. 79IECh. 18 - Prob. 80IECh. 18 - Prob. 81IECh. 18 - Prob. 82IECh. 18 - Prob. 83IECh. 18 - Prob. 84IECh. 18 - 18.85 The main reason that distillation is a...Ch. 18 - Prob. 86IECh. 18 - Prob. 87IECh. 18 - Prob. 88IECh. 18 - Prob. 89IECh. 18 - Prob. 90IECh. 18 - Prob. 91IECh. 18 - Prob. 92IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe the saline effect that occurs in solutions.arrow_forwardBriefly explain what the infinite dilution rate constant (k∞) consists of.arrow_forwardThe Davies equation corrects the Debye-Hückel limiting law for calculating the activity coefficient of an electrolyte in solution at relatively high concentrations. Mathematically, it is expressed as: log y₁ = -Az²? 1 + √Ĩ - 0,31) Is the formula correct?arrow_forward
- Differentiate between the concepts of "ionic salt effect" and "kinetic salt effect."arrow_forwardDifferentiate the concepts of “salino effect” and “salino kinetic effect”.arrow_forwardCome and compare the Bronsted-Bjerrum calculation, the Debye and Hückel calculation, and the Davies calculation.arrow_forward
- plz watch the youtube video (the title of this topic) by roxi H. she explains it step by step but i get the wrong answerarrow_forwardWriting the rate law implied by a simple mechanism To exit full screen, press and hold esc Suppose the decomposition of ozone proceeds by the following mechanism: step elementary reaction rate constant 1 →>> O3(9) O2(g) + O(g) k₁ 2 03(g) + O(g) → 202(g) k2 Suppose also k₁ »k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. ☐ rate = ☐ Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of K1, K2, and (if necessary) the rate constants k-1 and K-2 for the reverse of the two elementary reactions in the mechanism. k = ☐ 000 18 ローロ Ar OOarrow_forwardDeducing a rate law from the change in concentration over time To exit full screen, press and hold esc A chemistry graduate student is studying the rate of this reaction: H2CO3(aq) → H₂O(aq) +CO₂ (aq) - She fills a reaction vessel with H2CO3 and measures its concentration as the reaction proceeds: time (milliseconds) [H2CO3] 0 0.0500 M 10. 0.0266M 20. 0.0181 M 30. 0.0138M 40. 0.0111 M Use this data to answer the following questions. Write the rate law for this reaction. Calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. rate ☐ x10 k = Х 000 18 Ararrow_forward
- Writing the rate law implied by a simple mechanism Suppose the formation of tert-butanol proceeds by the following mechanism: step elementary reaction 1 (CH3)3 CBr(aq) → (CH3)2 C* (aq) + Br (aq) 2 (CH3)2C (aq) + OH¯ (aq) → (CH3)2COH(aq) rate constant k₁ k₂ Suppose also k₁ »k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. Note: your answer should not contain the concentrations of any intermediates. rate = k ☐ Express the rate constant k for the overall chemical reaction in terms of K1, K2, and (if necessary) the rate constants k-1 and K-2 for the reverse of the two elementary reactions in the mechanism. k = ☐ □ ☑ G ? 00. 18 Ar Barrow_forwardDeducing a rate law from the change in concentration over time A chemistry graduate student is studying the rate of this reaction: 2SO3 (g) →>> 2SO2 (g) + O2(g) He fills a reaction vessel with SO3 and measures its concentration as the reaction proceeds: ? time (minutes) [SO3] 0 0.0200M 1.0 0.0105 M 2.0 0.00552M 3.0 0.00290M 4.0 0.00152M Use this data to answer the following questions. Write the rate law for this reaction. rate = k ☐ x10 Calculate the value of the rate constant k. Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. k = ☐ Х 000 18 Ar BAarrow_forwardUsing the Arrhenius equation to calculate k at one temperature from k at... The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E reaction is 1.2 × 107 M −1 .S at 160.0 °C, what will the rate constant be at 194.0 °C? Round your answer to 2 significant digits. k = Шм −1 -1 .S ☐ x10 ☑ 5 = = 16.0 kJ/mol. If the rate constant of this a ? olo Ar Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY