
a) Styrene (PhCH=CH2)
Interpretation:
How to prepare styrene from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield
To state:
How to prepare styrene from 2-phenylethanol.
Answer to Problem 47AP
Styrene can be prepared by treating 2-phenylethanol with POCl3 in pyridine.
Explanation of Solution
2-Phenylethanol when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene.
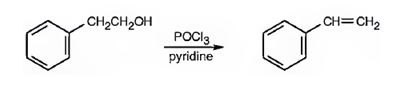
Styrene can be prepared by treating 2-phenylethanol with POCl3 in pyridine.
b) Phenylacetaldehyde (PhCH2CHO)
Interpretation:
How to prepare phenylacetaldehyde from 2-phenylethanol is to be stated.
Concept introduction:
Dess-Martin periodinate in dichloromethane oxidizes 10alcohols to
To state:
How to prepare phenylacetaldehyde from 2-phenylethanol
Answer to Problem 47AP
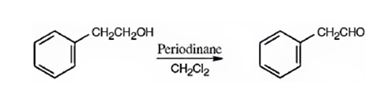
Phenylacetaldehyde can be prepared by oxidizing 2-phenylethanol with Dess-Martin periodinate in dichloromethane.
Explanation of Solution
2-phenylethanol is a 10 alcohol. It gets oxidized to phenylacetaldehyde when treated with Dess-Martin periodinate in dichloromethane.
Phenylacetaldehyde can be prepared by oxidizing 2-phenylethanol with Dess-Martin periodinate in dichloromethane.

c) Phenylacetic acid (PhCH2COOH)
Interpretation:
How to prepare phenylacetic acid from 2-phenylethanol is to be stated.
Concept introduction:
CrO3 in acidic solutions oxidize 10 alcohols directly into acids and 20 alcohols to ketones. It does not oxidize 30 alcohols.
To state:
How to prepare phenylacetic acid from 2-phenylethanol.
Answer to Problem 47AP
Phenylacetic acid can be prepared by oxidizing 2-phenylethanol with CrO3 in acidic solutions.
Explanation of Solution
2-phenylethanol is a 10 alcohol. It gets oxidized to phenylacetic acid when treated with CrO3 in acidic solutions.
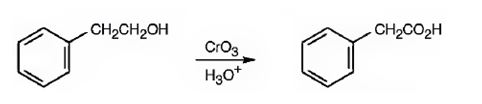
Phenylacetic acid can be prepared by oxidizing 2-phenylethanol with CrO3 in acidic solutions.
d) Benzoic acid
Interpretation:
How to prepare benzoic acid from 2-phenylethanol is to be stated.
Concept introduction:
KMnO4 in acidic solutions oxidize
To state:
How to prepare benzoic acid from 2-phenylethanol.
Answer to Problem 47AP
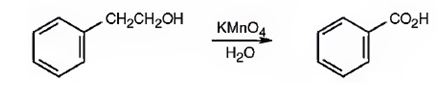
Benzoic acid can be prepared by oxidizing 2-phenylethanol with KMnO4 in acidic solutions.
Explanation of Solution
2-phenylethanol is a 10 alcohol. It gets oxidized to benzoic acid when treated with KMnO4 in acidic solutions.
Benzoic acid can be prepared by oxidizing 2-phenylethanol with KMnO4 in acidic solutions.
e) Ethylbenzene
Interpretation:
How to prepare ethylbenzene from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield an alkene. The alkene upon reduction gives the
To state:
How to prepare ethylbenzene from 2-phenylethanol.
Answer to Problem 47AP
Ethylbenzene can be prepared from 2-phenylethanol by following the steps shown below.
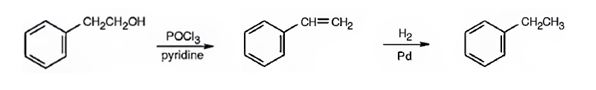
Explanation of Solution
2-Phenylethanol when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene. When treated with H2/Pd, the double bond in the side chain gets reduced to yield ethyl benzene.
Ethylbenzene can be prepared from 2-phenylethanol by following the steps shown below.

f) benzaldehyde
Interpretation:
How to prepare benzaldehyde from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield an alkene. The alkene upon ozonolyzis followed by reduction will yield the aldehyde required.
To state:
How to prepare benzaldehyde from 2-phenylethanol.
Answer to Problem 47AP
Benzaldehyde can be prepared from 2-phenylethanol by following the steps shown below.
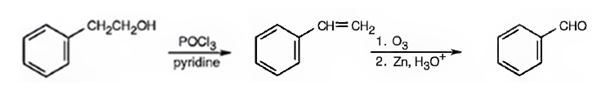
Explanation of Solution
2-Phenylethanol when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene. When styrene is subjected to ozonolysis followed by reduction, the double bond in side chain gets cleaved resulting in the formation of benzaldehyde.
Benzaldehyde can be prepared from 2-phenylethanol by following the steps shown below.

g) 1-phenylethanol
Interpretation:
How to prepare 1-phenylethanol from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols undergo dehydration when treated with POCl3 in pyridine to yield an alkene. The alkene adds a molecule of water following oxymercuration-demercuration process. The addition will take place following Markovnikov regiochemistry.
To state:
How to prepare 1-phenylethanol from 2-phenylethanol.
Answer to Problem 47AP
1- Phenylethanol can be prepared from 2-phenylethanol by following the steps shown below.
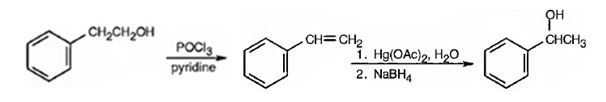
Explanation of Solution
2-Phenylethanol, when treated with POCl3 in pyridine eliminates a molecule of water from the side chain to yield styrene. When styrene is subjected oxymercuration-demercuration processes, a molecule of water is added, following Markovnikov regiochemistry, to the double bond. The –OH adds on to the more alkyl substituted carbon and H to the less alkyl substituted carbon in double bond to yield 1-phenylethanol.
1-Phenylethanol can be prepared from 2-phenylethanol by following the steps shown below.

h) 1-Bromo-2-phenylethane
Interpretation:
How to prepare 1-bromo-2-phenylethane from 2-phenylethanol is to be stated.
Concept introduction:
Alcohols yield the corresponding alkyl bromides when treated with PBr3.
To state:
How to prepare 1-bromo-2-phenylethane from 2-phenylethanol is to be stated.
Answer to Problem 47AP
1-Bromo-2-phenylethane can be prepared from 2-phenylethanol by following the steps shown below.
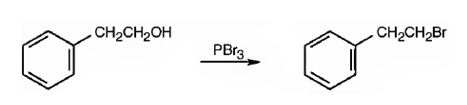
Explanation of Solution
When 2-phenylethanol is treated with PBr3, a bimolecular nucleophilic substitution of –OH by Br takes place to yield 1-bromo-2-phenylethane.
1-Bromo-2-phenylethane can be prepared from 2-phenylethanol by following the steps shown below.

Want to see more full solutions like this?
Chapter 17 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- Assign ALL signals for the proton and carbon NMR spectra on the following pages.arrow_forward7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forwardAssign the functional group bands on the IR spectra.arrow_forward
- Find the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forward
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





