
Physics (5th Edition)
5th Edition
ISBN: 9780321976444
Author: James S. Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 56PCE
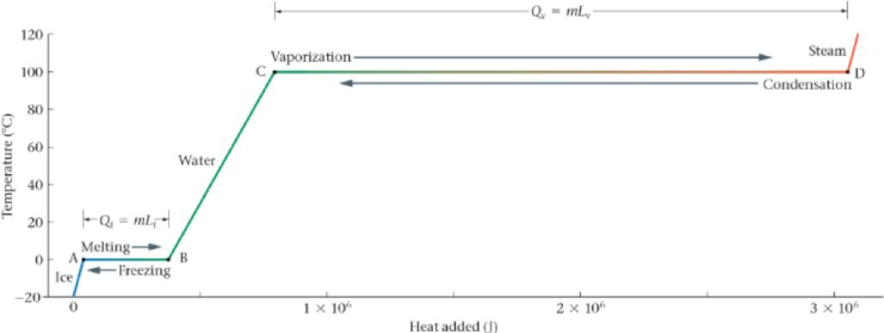
Figure 17-30 shows a temperature-versus-heat plot for 1.000 kg of water. (a) Calculate the heat corresponding to the points A, B, C, and D. (b) Calculate the slope of the line from point B to point C. Show that this slope is equal to 1/c, where c is the specific heat of liquid water.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In very cold weather, a significant mechanism for heat loss by the human body is the energy expended in warming the air taken into the lungs with each breath. (a) On a cold winter day when the temperature is - 20 ℃, what amount of heat is needed to warm body temperature (37 ℃) the 0.60 L of air exchanged with each breath? Assume that the specific heat of air is 1020 J/kg.K and that 1.0 L of air has a mass of 1.3 x 10-3 kg. (b) How much heat is lost per hour if the respiration rate is 20 breaths per minute?
Radioactive decay of elements in the earth’s interior results in a mean heat flux through the Earth’s surfaceof 5×10−2Wm−2. What is the flux expressed as a fraction of the energy flux due to thermal re-radiation of absorbed solar energy? If radioactive decay were the only heat source for the Earth, what would the Earth’s temperature be?
A food product containing 80% moisture content is being frozen. Estimate the specific heat of the product at -6 ° C when 80% of the water is frozen. The specific heat of the dry product is 2 kJ / (kg ° C). it is assumed that the specific heat of water at -10 ° C is the same as the specific heat of water at 0 ° C, and the specific heat of ice follows the function Cp ice = 0.0062 Freezing point + 2.0649.
Cp of frozen product = .... kJ / kg ° C.
Chapter 17 Solutions
Physics (5th Edition)
Ch. 17.1 - Rank the following ideal-gas systems in order of...Ch. 17.2 - If the Kelvin temperature of a gas is doubled, by...Ch. 17.3 - A metal rod of a given initial length and...Ch. 17.4 - A portion of a substances phase diagram is shown...Ch. 17.5 - Which requires more heat: melting 100 kg of copper...Ch. 17.6 - An ice cube is placed in a cup of water. A few...Ch. 17 - How is the air pressure in a tightly sealed house...Ch. 17 - The average speed of air molecules in your room is...Ch. 17 - Is it possible to change both the pressure and the...Ch. 17 - Prob. 4CQ
Ch. 17 - A camping stove just barely boils water on a...Ch. 17 - An autoclave is a device used to sterilize medical...Ch. 17 - As the temperature of ice is increased, it changes...Ch. 17 - BIO Isopropyl alcohol is sometimes rubbed onto a...Ch. 17 - A drop of water on a kitchen counter evaporates in...Ch. 17 - (a) Is the number of molecules in one mole of N2...Ch. 17 - Predict/Explain If you put a helium-filled balloon...Ch. 17 - Two containers hold ideal gases at the same...Ch. 17 - Prob. 4PCECh. 17 - BIO After emptying her lungs, a person inhales 4.3...Ch. 17 - An automobile tire has a volume of 0.0185 m3. At a...Ch. 17 - Prob. 7PCECh. 17 - A compressed-air tank holds 0.500 m3 of air at a...Ch. 17 - Four ideal gases have the following pressures, P,...Ch. 17 - A balloon contains 3.9 liters of nitrogen gas at a...Ch. 17 - Prob. 11PCECh. 17 - Predict/Calculate A bicycle tire with a volume of...Ch. 17 - A 515-cm3 flask contains 0.460 g of a gas at a...Ch. 17 - Prob. 14PCECh. 17 - The air inside a hot-air balloon has an average...Ch. 17 - Prob. 16PCECh. 17 - Consider the system described in the previous...Ch. 17 - Prob. 18PCECh. 17 - Prob. 19PCECh. 17 - If the translational speed of molecules in an...Ch. 17 - At what temperature is the rms speed of H2 equal...Ch. 17 - Suppose a planet has an atmosphere of pure ammonia...Ch. 17 - Prob. 23PCECh. 17 - Prob. 24PCECh. 17 - Prob. 25PCECh. 17 - What is the temperature of a gas of CO2 molecules...Ch. 17 - The rms speed of a sample of gas is increased by...Ch. 17 - Prob. 28PCECh. 17 - A 380-mL spherical flask contains 0.065 mol of an...Ch. 17 - Prob. 30PCECh. 17 - A rock climber hangs freely from a nylon rope that...Ch. 17 - BIO To stretch a relaxed biceps muscle 2.5 cm...Ch. 17 - A 22-kg chimpanzee hangs from the end of a...Ch. 17 - The Marianas Trench The deepest place in all the...Ch. 17 - Four cylindrical rods with various cross-sectional...Ch. 17 - Predict/Calculate A steel wire 4.1 m long...Ch. 17 - BIO Spiderweb An orb weaver spider with a mass of...Ch. 17 - Predict/Calculate Two rods of equal length (0.55...Ch. 17 - A piano wire 0.82 m long and 0.93 mm in diameter...Ch. 17 - The formation of ice from water is accompanied by...Ch. 17 - Vapor Pressure for Water Figure 17-35 shows a...Ch. 17 - Using the vapor-pressure curve given in Figure...Ch. 17 - Prob. 43PCECh. 17 - Prob. 44PCECh. 17 - Predict/Calculate The Vapor Pressure of CO2 A...Ch. 17 - Phase Diagram for Water The phase diagram for...Ch. 17 - Phase Diagram for CO2 The phase diagram for CO2 is...Ch. 17 - Prob. 48PCECh. 17 - How much heat must be removed from 1.96 kg of...Ch. 17 - A heat transfer of 9.5 105 J is required to...Ch. 17 - How much heat must be added to 2.55 kg of copper...Ch. 17 - An ammonia refrigeration cycle involves the...Ch. 17 - Prob. 53PCECh. 17 - Prob. 54PCECh. 17 - Prob. 55PCECh. 17 - Figure 17-30 shows a temperature-versus-heat plot...Ch. 17 - Predict/Calculate Suppose the 1.000 kg of water in...Ch. 17 - Prob. 58PCECh. 17 - When you go out to your car one cold winter...Ch. 17 - A large punch bowl holds 3.99 kg of lemonade...Ch. 17 - A 155-g aluminum cylinder is removed from a liquid...Ch. 17 - An 825-g iron block is heated to 352 C and placed...Ch. 17 - Party Planning You are expecting to serve 32 cups...Ch. 17 - Predict/Calculate A 35-g ice cube at 0.0 C is...Ch. 17 - A 48-g block of copper at 12 C is added to 110 g...Ch. 17 - A 0 075-kg ice cube at 0.0 C is dropped into a...Ch. 17 - To help keep her barn warm on cold days, a farmer...Ch. 17 - CE As you go up in attitude, do you expect the...Ch. 17 - Prob. 69GPCh. 17 - Prob. 70GPCh. 17 - Prob. 71GPCh. 17 - Cooling Computers Researchers are developing heat...Ch. 17 - Prob. 73GPCh. 17 - Prob. 74GPCh. 17 - Evaporating Atmosphere Hydrogen gas evaporates...Ch. 17 - Prob. 76GPCh. 17 - A Boiling Geyser (a) The column of water that...Ch. 17 - A Melting Glacier (a) A glacier is made of ice of...Ch. 17 - Peter catches a 4 2-kg striped bass on a fishing...Ch. 17 - A steel ball (density=7860kg/m3) with a diameter...Ch. 17 - A lead brick with the dimensions shown in Figure...Ch. 17 - (a) Find the amount of heat that must be extracted...Ch. 17 - Mighty Ice Lift A tremendous force is generated...Ch. 17 - Orthopedic Implants Metals such as titanium and...Ch. 17 - Students on a spring break picnic bring a cooler...Ch. 17 - A 5.9-kg block of ice at 1.5 C slides on a...Ch. 17 - A cylindrical copper rod 37 cm long and 7.5 cm in...Ch. 17 - Prob. 88PPCh. 17 - Prob. 89PPCh. 17 - Prob. 90PPCh. 17 - Prob. 91PPCh. 17 - Referring to Example 17-17 (a) Find the final...Ch. 17 - Referring to Example 17-17 (a) Find the final...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Choose the best answer to each of the following. Explain your reasoning. Which of the following three kinds of ...
Cosmic Perspective Fundamentals
What class of motion, natural or violent, did Aristotle attribute to motion of the Moon?
Conceptual Physics (12th Edition)
In lightning storms, the potential difference between the Earth and the bottom of the thunderclouds can be as h...
Physics for Scientists and Engineers with Modern Physics
27. A pendulum has a period of 1.85 s on Earth. Whatis its period on Mars, where the acceleration of gravity is...
Physics: Principles with Applications
54. If a person has a dangerously high fever, submerging her in ice water is a bad idea, but an ice pack can he...
College Physics: A Strategic Approach (3rd Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
The Cosmic Perspective Fundamentals (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- For a temperature increase of 10 at constant volume, what is the heat absorbed by (a) 3.0 mol of a dilute monatomic gas; (b) 0.50 mol of a dilute diatomic gas; and (c) 15 mol of a dilute polyatomic gas?arrow_forwardOne of a dilute diatomic gas occupying a volume of 10.00 L expands against a constant pressure of 2.000 atm when it is slowly heated. If the temperature of the gas rises by 10.00 K and 400.0 J of heat are added in the process, what is its final volume?arrow_forward(a) What is the rate of heat conduction through the 3.00-cm-thick fur of a large animal having a I .40-m surface area? Assume that the animal's skin temperature is 32.0 , that the air temperature is 5.00 , and that has the same thermal conductivity as air. (b) What food intake will the animal need in one day to replace this heat transfer?arrow_forward
- A hermetically sealed room 4 m x 4 m x 4 m has an internal sensible heat gain of 3 kW.The overall heat – transfer coefficient of its wall, floor and ceiling is 1.1 W/m2K. Theoutside temperature is constant at 37 °C. Calculate the steady – state temperature of theroom. What will be the temperature maintained inside the room if the ventilation airsupplied is equivalent to 10 air changes per hour?arrow_forwardThe cross section radius of a bar of metal of length L and uniform coefficient of thermal conductivity k is expressed as r = (1 + x)1/2, where x is the distance from one end of the bar. Calculate the rate of heat transfer H if the two ends are kept at temperatures TH and TL respectivelyarrow_forward(a) Calculate the rate of heat conduction (in W) through house walls that are 11.5 cm thick and that have an average thermal conductivity twice that of glass wool. Assume there are no windows or doors. The surface area of the walls is 150 m2 and their inside surface is at 20.0°C, while their outside surface is at 5.00°C. Answer _________ W (NO scientific notation ONLY Real Number) (b) How many 1 kW room heaters would be needed to balance the heat transfer due to conduction? (Round your answer to the next whole integer.) Answer__________ 1 kW room heaters (NO scientific notation ONLY Real Number)arrow_forward
- Ex(1): What is the amount of heat energy necessary to raise temperature ( 3 kg ) of Aluminum from ( 15 c °) to ( 25 c °) the specific heat of Aluminum (900 J/kg. c°).arrow_forward(a) What is the rate of heat conduction through the 3.00-cm-thick fur of a large animal having a 1.40-m2 surface area? Assume that the animal’s skin temperature is 32.0ºC , that the air temperature is −5.00ºC , and that fur has the same thermal conductivity as air. (b) What food intake will the animal need in one day to replace this heat transfer?arrow_forwardProblem 1: (a) Large beds of rocks are used in some solar-heated homes to store heat. Assume that the specific heat of the rocks is 0.82 J/g-K. Calculate the quantity of heat absorbed by 50.0 kg of rocks if their temperature increases by 12.0 °C. (b) What temperature change would these rocks undergo if they emitted 450 kJ of heat?arrow_forward
- (a) Two 33 g ice cubes are dropped into 180 g of water in a thermally insulated container. If the water is initially at 27°C, and the ice comes directly from a freezer at -21°C, what is the final temperature at thermal equilibrium? (b) What is the final temperature if only one ice cube is used? The specific heat of water is 4186 J/kg-K. The specific heat of ice is 2220 J/kg•K. The latent heat of fusion is 333 kJ/kg. (a) Number Units (b) Number Unitsarrow_forwardHow much heat per second (H = Q/ΔT) is lost from the house due to heat conduction? The answer in watt must be rounded to the nearest 10 W.arrow_forwardPlease help mearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY