
ORGANIC CHEMISTRY-ETEXT REG ACCESS
12th Edition
ISBN: 9781119308362
Author: Solomons
Publisher: WILEY
expand_more
expand_more
Solutions are available for other sections.
Concept explainers
Textbook Question
Chapter 17, Problem
Practice Problem 17.4
Show how each of the following compounds could be converted to benzoic acid:
(a)
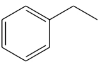
(b)
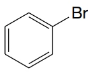
(c)
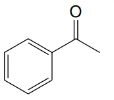
(d)
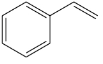
(e) Benzyl alcohol
(f) Benzaldehyde
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.
Write the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcohol
What type of interaction would you expect between the following R groups in the tertiary
structure of a protein?
O
-CH2-CO and -CH2-CH2-CH2-CH2-NH3+
a. disulfide bonds
b. salt bridges
c. hydrogen bonds
HO
abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT
d. hydrophobic interactions
e. peptide bonds
Chapter 17 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
Ch. 17 - Practice Problem 17.1 Give an IUPAC systematic...Ch. 17 - Prob. 2PPCh. 17 - Practice Problem 17.3 Write structural formulas...Ch. 17 - Practice Problem 17.4
Show how each of the...Ch. 17 - Practice Problem 17.5
Show how you could prepare...Ch. 17 - Practice Problem 17.6
(a) Which of the carboxylic...Ch. 17 - Prob. 7PPCh. 17 - Prob. 8PPCh. 17 - Practice Problem 17.9
Esters can also be...Ch. 17 - Prob. 10PP
Ch. 17 - Prob. 11PPCh. 17 - Practice Problem 17.12
What products would you...Ch. 17 - Practice Problem 17.13 (a) Provide the reagents...Ch. 17 - Prob. 14PPCh. 17 - Practice Problem 17.15 Using decarboxylation...Ch. 17 - Practice Problem 17.16 Diacyl peroxides, ,...Ch. 17 - Prob. 17PCh. 17 - Give an IUPAC systematic or common name for each...Ch. 17 - Prob. 19PCh. 17 - Prob. 20PCh. 17 - 17.21 What major organic product would you expect...Ch. 17 - Prob. 22PCh. 17 - Prob. 23PCh. 17 - Prob. 24PCh. 17 - Prob. 25PCh. 17 - 17.26 What products would you expect to obtain...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - 17.28 Indicate reagents that would accomplish each...Ch. 17 - Write structural formulas for the major organic...Ch. 17 - Prob. 30PCh. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - 17.33 On heating,...Ch. 17 - Prob. 34PCh. 17 - Prob. 35PCh. 17 - 17.36 Show how pentanoic acid can be prepared from...Ch. 17 - 17.37 The active ingredient of the insect...Ch. 17 - Prob. 38PCh. 17 - Prob. 39PCh. 17 - Give stereochemical formulas for compounds AQ:...Ch. 17 - 17.41 -Glyceraldehyde can be transformed into...Ch. 17 - Prob. 42PCh. 17 - Prob. 43PCh. 17 - 17.44 Given here are the NMR spectra and carbonyl...Ch. 17 - 17.45 Compound Y dissolves slowly when warmed...Ch. 17 - Prob. 46PCh. 17 - Prob. 47PCh. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - 17.52 Starting with 1-naphthol, suggest an...Ch. 17 - Suggest a synthesis of ibuprofen (Section 5.11)...Ch. 17 - Prob. 54PCh. 17 - Prob. 55PCh. 17 - Prob. 1LGPCh. 17 - Prob. 2LGPCh. 17 - Prob. 3LGPCh. 17 - Prob. 4LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
While driving a car at 90 km/h, how far do you move while your eyes shut for 0.50 s during a hard sneeze?
Fundamentals of Physics Extended
1.6 Read the labels on products used to wash your dishes. What are the names of some chemicals contained in tho...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
27. Consider the reaction.
Express the rate of the reaction in terms of the change in concentration of each of...
Chemistry: Structure and Properties (2nd Edition)
Where is transitional epithelium found and what is its importance at those sites?
Anatomy & Physiology (6th Edition)
DRAW IT The diagram shows a cell in meiosis. (a) Label the appropriate structures with these terms: chromosome ...
Campbell Biology (11th Edition)
Chlorine has two isotopes, 35Cl and 37Cl; 75.77% of chlorine is 35Cl, and 24.23% is 37Cl. The atomic mass of 35...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY