
(a)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 1. Identify and name the parent
- 2. Identify and name the substituent
- 3. Assign a locant to each substituent
- 4. Arrange the substituent alphabetically
(a)
Answer to Problem 24PP
- Name for the given compound
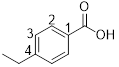
4-ethylbenzoic acid
Explanation of Solution
4-ethylbenzoic acid
From the structure of the compound it is clear that the parent group is benzoic acid.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number, for the given compound an ethyl group is a substituent and it is placed to the 4th (
(b)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 5. Identify and name the parent
- 6. Identify and name the substituent
- 7. Assign a locant to each substituent
- 8. Arrange the substituent alphabetically
(b)
Answer to Problem 24PP
- Name for the given compound
b)
-
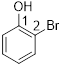
2-bromophenol
Explanation of Solution
The given compound b2-bromophenol
From the structure of the compound it is clear that the parent group is phenol.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound bromine atom is a substituent and they are placed to the 2nd (
(c)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 9. Identify and name the parent
- 10. Identify and name the substituent
- 11. Assign a locant to each substituent
- 12. Arrange the substituent alphabetically
(c)
Answer to Problem 24PP
- Name for the given compound
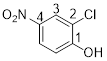
2-chloro-4-nitrophenol
Explanation of Solution
The given compound c
2-chloro-4-nitrophenol
From the structure of the compound it is clear that the parent group is phenol.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound a chlorine atom and nitro group are substituent and they are placed to the 2nd (
(d)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 13. Identify and name the parent
- 14. Identify and name the substituent
- 15. Assign a locant to each substituent
- 16. Arrange the substituent alphabetically
(d)
Answer to Problem 24PP
- Name for the given compound
d)
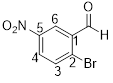
2-bromo-5-nitrobenzaldehyde
Explanation of Solution
The given compound d
2-bromo-5-nitrobenzaldehyde
From the structure of the compound it is clear that the parent group is benzaldehyde.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound a bromine atom and nitro group are substituent and they are placed to the 2nd (
(e)
Interpretation: For the given compounds, IUPAC name should be provided.
Concept introduction:
For naming and the polysubstituted benzene ring, following steps should be followed.
- 17. Identify and name the parent
- 18. Identify and name the substituent
- 19. Assign a locant to each substituent
- 20. Arrange the substituent alphabetically
(e)
Answer to Problem 24PP
- Name for the given compound
-
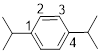
1, 4-diisopropylbenzene
Explanation of Solution
The given compound e
1, 4-diisopropylbenzene
From the structure of the compound it is clear that the parent group is benzene.
The second step to name the structure of the polysubstituted benzene ring is
Identification of substituent and its locant at the lowest possible number. For the given compound there are two substituents, both isopropyl group it is placed to the 1st (
Want to see more full solutions like this?
Chapter 17 Solutions
ORGANIC CHEMISTRY LL PRINT UPGRADE
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





