
Chemistry (OER)
2nd Edition
ISBN: 9781947172616
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 19E
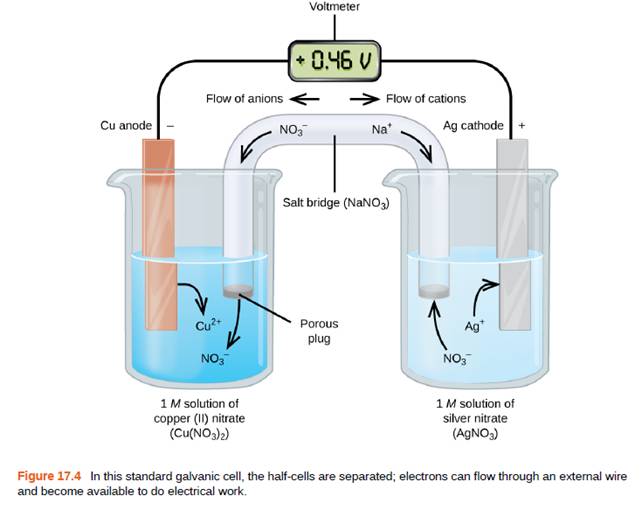
Why is a salt bridge necessary in galvanic cells like the one in Figure 17.4?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 17 Solutions
Chemistry (OER)
Ch. 17 - If a 2.5 A current is run through a circuit for 35...Ch. 17 - For the scenario in the previous question, how...Ch. 17 - For each of the following balanced half-reactions,...Ch. 17 - For each of the following balanced half-reactions,...Ch. 17 - Given the following pairs of balanced...Ch. 17 - Balance the following in acidic solution: (a)...Ch. 17 - Identify the species that undergoes oxidation, the...Ch. 17 - Balance the following in acidic solution: (a)...Ch. 17 - Identify the species that was oxidized, the...Ch. 17 - Why is it not possible for hydroxide ion (OH-) to...
Ch. 17 - Why is it not possible for hydrogen ion (H+) to...Ch. 17 - Why must the charge balance in oxidation-reduction...Ch. 17 - Write the following balanced reactions using cell...Ch. 17 - Given the following cell notations, determine the...Ch. 17 - For the cell notations in the previous problem,...Ch. 17 - Balance the following reactions and write the...Ch. 17 - Identify the species oxidized species reduced, and...Ch. 17 - From the information provided, use cell notation...Ch. 17 - Why is a salt bridge necessary in galvanic cells...Ch. 17 - An active (metal) electrode was found to gain mass...Ch. 17 - An active (metal) electrode was found to lose mass...Ch. 17 - The mass of three different metal electrodes, each...Ch. 17 - For each reaction listed, determine its standard...Ch. 17 - For each reaction listed, determine its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - Determine the overall reaction and its standard...Ch. 17 - For the standard cell potentials given here,...Ch. 17 - For the ?G values given here, determine the...Ch. 17 - Determine the standard cell potential and the cell...Ch. 17 - Determine G and G for each of the reactions in...Ch. 17 - Use the data in Appendix L to determine the...Ch. 17 - What are the desirable qualities of an electric...Ch. 17 - List some things that are typically considered...Ch. 17 - Consider a battery made from one half-cell that...Ch. 17 - Consider a battery with the overall reaction:...Ch. 17 - An inventor proposes using a SHE (standard...Ch. 17 - Why do batteries go dead, but fuel cells do not?Ch. 17 - Explain what happens to battery voltage as a...Ch. 17 - Using the information thus far in this chapter,...Ch. 17 - Which member of each pair of metals is more likely...Ch. 17 - Consider the following metals: Ag, Au, Mg, Ni, and...Ch. 17 - Aluminum (E Al 3+/Al=2.07V) is more easily...Ch. 17 - If a sample of iron and a sample of zinc come into...Ch. 17 - Suppose you have three different metals. A, B, and...Ch. 17 - Why would a sacrificial anode made of lithium...Ch. 17 - Identify the reaction at the anode, reaction at...Ch. 17 - What mass of each product is produced in each of...Ch. 17 - How long would it take to reduce 1 mole of each of...Ch. 17 - A current of 2.345 A passes through the cell shown...Ch. 17 - An irregularly shaped metal part made from a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match the following examples of mutagens. Column A Column B ___a. A mutagen that is incorporated into DNA in pl...
Microbiology: An Introduction
According to the logistic growth equation dNdt=rN(KN)K (A) the number of individuals added per unit time is gre...
Campbell Biology (11th Edition)
1. What are the main organs of the skeletal system?
Human Anatomy & Physiology (2nd Edition)
In your own words, briefly distinguish between relative dates and numerical dates.
Applications and Investigations in Earth Science (9th Edition)
1. A person gets in an elevator on the ground floor and rides it to the top floor of a building. Sketch a veloc...
College Physics: A Strategic Approach (3rd Edition)
Choose the more metallic element from each pair. a. Sb or Pb b. K or Ge c. Ge or Sb d. As or Sn
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Introduction to Electrochemistry; Author: Tyler DeWitt;https://www.youtube.com/watch?v=teTkvUtW4SA;License: Standard YouTube License, CC-BY