
Organic Chemistry Third Edition + Electronic Solutions Manual And Study Guide
3rd Edition
ISBN: 9781119351610
Author: David Klein
Publisher: Wiley Plus
expand_more
expand_more
format_list_bulleted
Question
Chapter 16.5, Problem 11ATS
Interpretation Introduction
Interpretation: The products of the given reaction have to be determined and also have to predict which product will predominate under
Concept Introduction:
Electrophilic addition of
In the first step, most stable carbocation is formed and then it is subjected to nucleophilic attack leading to two different products. The products are called 1,2-adduct and 1,4-adduct.
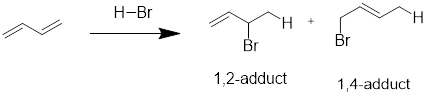
The pathway which occur rapidly is the 1,2-addition and the reaction is said to be under kinetic control.
The 1,4-adduct is lower in energy and the reaction is said to be under thermodynamic control.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the temperature change produced by burning 3.55 g benzoic acid in a bomb calorimeter that has a heat capacity of 20.12 kJ/°C. The enthalpy of combustion of benzoic acid is −26.43 kJ/g.
Determine the entropy change for the reaction SO 2 (g) + O2(g) → SO3(g) given the following
information:
Substance
S° (J/mol K)
.
SO2(g)
248.2
O2(g)
205.0
SO3(g)
256.8
Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water. If ΔH ° = −56.13 kJ/mol and ΔS ° = 79.11 J/mol ⋅ K, what is the temperature of the reaction if ΔG ° = −80.89 kJ/mol?
Chapter 16 Solutions
Organic Chemistry Third Edition + Electronic Solutions Manual And Study Guide
Ch. 16.1 - Prob. 1CCCh. 16.2 - Prob. 2CCCh. 16.2 - Prob. 3CCCh. 16.2 - Prob. 4CCCh. 16.3 - Prob. 5CCCh. 16.4 - Prob. 1LTSCh. 16.4 - Prob. 6PTSCh. 16.4 - Prob. 7PTSCh. 16.4 - Prob. 8ATSCh. 16.5 - Prob. 2LTS
Ch. 16.5 - Prob. 9PTSCh. 16.5 - Prob. 10PTSCh. 16.5 - Prob. 11ATSCh. 16.5 - Prob. 12CCCh. 16.7 - Prob. 3LTSCh. 16.7 - Prob. 13PTSCh. 16.7 - Prob. 14ATSCh. 16.7 - Prob. 15CCCh. 16.7 - Prob. 16CCCh. 16.7 - Prob. 17CCCh. 16.7 - Predict the regiochemical outcome (major product)...Ch. 16.8 - Prob. 19CCCh. 16.9 - Prob. 20CCCh. 16.9 - Prob. 4LTSCh. 16.9 - Prob. 21PTSCh. 16.9 - Prob. 22ATSCh. 16.10 - Prob. 23CCCh. 16.10 - Prob. 24CCCh. 16.10 - Prob. 25CCCh. 16.10 - Prob. 26CCCh. 16.11 - Prob. 5LTSCh. 16.11 - Prob. 27PTSCh. 16.11 - Prob. 28ATSCh. 16.12 - Prob. 29CCCh. 16 - Prob. 30PPCh. 16 - Prob. 31PPCh. 16 - Prob. 32PPCh. 16 - Prob. 33PPCh. 16 - Prob. 34PPCh. 16 - Prob. 35PPCh. 16 - Prob. 36PPCh. 16 - Prob. 37PPCh. 16 - Prob. 38PPCh. 16 - Prob. 39PPCh. 16 - Prob. 40PPCh. 16 - Prob. 41PPCh. 16 - Prob. 42PPCh. 16 - Prob. 43PPCh. 16 - Prob. 44PPCh. 16 - Prob. 45PPCh. 16 - Prob. 46PPCh. 16 - Prob. 47PPCh. 16 - Prob. 48PPCh. 16 - Prob. 49PPCh. 16 - Prob. 50PPCh. 16 - Prob. 51PPCh. 16 - Prob. 52PPCh. 16 - Prob. 53PPCh. 16 - Prob. 54PPCh. 16 - Prob. 55PPCh. 16 - Prob. 56PPCh. 16 - Prob. 57PPCh. 16 - Prob. 58PPCh. 16 - Prob. 59PPCh. 16 - Prob. 60IPCh. 16 - Prob. 61IPCh. 16 - Prob. 62IPCh. 16 - Prob. 63IPCh. 16 - Prob. 64IPCh. 16 - Prob. 65IPCh. 16 - Prob. 66IPCh. 16 - Prob. 67IPCh. 16 - Prob. 68IPCh. 16 - Prob. 69IPCh. 16 - Prob. 70IPCh. 16 - Prob. 71IPCh. 16 - Prob. 72IPCh. 16 - Prob. 73IPCh. 16 - Prob. 74IPCh. 16 - Prob. 76IPCh. 16 - Prob. 77CPCh. 16 - Prob. 78CPCh. 16 - Prob. 79CPCh. 16 - Prob. 80CPCh. 16 - Prob. 81CP
Knowledge Booster
Similar questions
- For a particular hypothetical reaction, A+B →2C, the value of AG° is -125 kJ/mol. What is the value of AG for this reaction at 35°C when [A] = 0.10 M, [B] = 0.05 M, and [C] = 2.0 × 10¹ M?arrow_forwardIn an experiment, 74.3 g of metallic copper was heated to 100.0°C and then quickly dropped into 200.0 mL of water in a calorimeter. The heat capacity of the calorimeter with the water was 875 J/°C. The initial temperature of the calorimeter was 27.5°C, and the final temperature after addition of the metal was 29.8°C. What is the value of the molar heat capacity of copper?arrow_forwardThe Haber-Bosch process permits the direct conversion of molecular nitrogen to ammonia, which can be used in large-scale fertilizer production. Given the balanced Haber-Bosch reaction and using the bond energies in the table below, estimate the enthalpy change associated with the reaction. N2(g) + 3H2(g) → 2NH3(g) Bond N=N N = N Energy (kJ/mol) 941 418 N-N H-H N-H 163 435 388arrow_forward
- Benzoic acid is used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form and its energy of combustion is known very accurately (−26.43 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 9.199°C when 3.500 g of benzoic acid was used.arrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 2N2H4(g) + 2NO2(g) → 3N2(g) + 4H2O(g) AHrxn ? kJ Substance AH in kJ/mol N2H4(g) +95.4 NO2(g) +33.1 H2O(g) -241.8arrow_forwardIf 7.3 kJ of energy are required to change the temperature of water from 5.0 to 70.0, what was the volume of water? (cs = 4.184 J/(g ⋅ ), d = 1.00 g/mL)arrow_forward
- BALANCE CHEMICAL REACTIONarrow_forwardPredict the product(s) of the following reactions. If no reaction, write "NR". a) Cl₂ FeCl3 e) HNO3 H2SO4 b) NO2 CI. HNO3 f) Br Br2 OH H2SO4 HO3S. FeBr3 c) Cl2 g) FeCl3 F d) O₂N Br2 FeBr3 O₂N OH HNO3 CH3 H2SO4arrow_forwardulating the pH salt solution Calculate the pH at 25 °C of a 0.75M solution of anilinium chloride (C6H5NH3C1). Note that aniline (C6H5NH2) is a weak base with a pK of 4.87. Round your answer to 1 decimal place. pH = ☐ ☑ ⑤ ? olo 18 Ararrow_forward
- I apologize, but the app is not allowing me to post the other 4 pictures of the thermodynamics chart. But I believe the values are universal. Please help!arrow_forwardCalculating the pH of a salt solution Calculate the pH at 25 °C of a 0.29M solution of potassium butanoate (KC3H,CO2). Note that butanoic acid (HC3H,CO2) is a weak acid with a pKa of 4.82. Round your answer to 1 decimal place. pH = -0 Х olo 18 Ararrow_forward: At a certain temperature, the equilibrium constant K for the following reaction is 1.58 × 10-12 N2(g) + O2(g) = 2 NO(g) Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.93 mol of N2 and 0.93 mol of O2. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and O2. There will be very little NO. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 NO(g) N2(9)+02(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 N2(9)+302(g) 6 NO(g) Neither of the above is true. K = ☐ K = ☐ ☐ X10 Х D ? 000 18 Ar Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY