
ORGANIC CHEMISTRY (LOOSELEAF)
6th Edition
ISBN: 9781260475630
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16.13, Problem 27P
Draw the products of each reaction.
a. 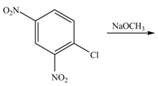 b.
b. 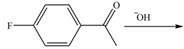
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?
14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing
150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3
Ag+ + S20
Ag(S203)¯
K₁ = 7.4 × 108
Ag(S203)¯ + S20¯ = Ag(S203)
K₂ = 3.9 x 104
ΗΝ,
cyclohexanone
pH 4-5
Draw Enamine
I
I
CH3CH2Br
THF, reflux
H3O+
I
Drawing
Draw Iminium Ion
Chapter 16 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)
Ch. 16.1 - Prob. 1PCh. 16.2 - Prob. 2PCh. 16.3 - Prob. 3PCh. 16.4 - Prob. 4PCh. 16.5 - Prob. 5PCh. 16.5 - Prob. 6PCh. 16.5 - Prob. 9PCh. 16.5 - Problem 18.9 Draw the product of each reaction
a....Ch. 16.5 - Prob. 11PCh. 16.5 - Prob. 12P
Ch. 16.5 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Problem 18.14 Draw all resonance structures for...Ch. 16.6 - Problem 18.15 Classify each substituent as...Ch. 16.7 - Prob. 17PCh. 16.9 - Prob. 22PCh. 16.10 - Problem 18.20 Draw the products of each...Ch. 16.10 - Prob. 24PCh. 16.11 - Problem 18.22 Draw the products formed when each...Ch. 16.12 - Problem 18.23 Devise a synthesis of each compound...Ch. 16.13 - Problem 18.24 Draw the products of each...Ch. 16.13 - Problem 18.25 Draw a stepwise mechanism for the...Ch. 16.13 - Problem 18.26 Draw the products of each...Ch. 16.13 - Prob. 30PCh. 16 - Prob. 37PCh. 16 - 18.35 What is the major product formed by an...Ch. 16 - 18.36 Draw the products formed when phenol is...Ch. 16 - Problem 18.37 Draw the products formed when each...Ch. 16 - 18.38 Draw the products of each reaction.
a. d....Ch. 16 - 18.39 What products are formed when benzene is...Ch. 16 - Prob. 49PCh. 16 - 18.47 For each of the following substituted...Ch. 16 - 18.48 Consider the tetracyclic aromatic compound...Ch. 16 - 18.49 For each N-substituted benzene, predict...Ch. 16 - Prob. 54PCh. 16 - 18.51 Using resonance structures, explain why a...Ch. 16 - Prob. 56PCh. 16 - 18.53 Rank the aryl halides in each group in order...Ch. 16 - Prob. 64PCh. 16 - Prob. 65PCh. 16 - Prob. 66PCh. 16 - 18.63 Synthesize each compound from benzene and...Ch. 16 - Problem 18.64 Synthesize each compound from...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward
- 14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY