
EBK ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260475685
Author: SMITH
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 67P
Synthesize each compound from benzene and any other organic or inorganic reagents.
a. 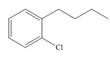 c.
c. 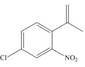 e.
e. 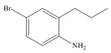 g.
g. 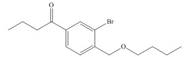
b. 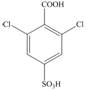 d.
d. 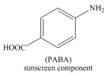 f.
f. 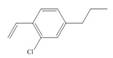 h.
h. 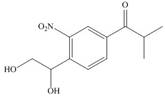
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Topic: Photochemistry and Photophysics of Supramolecules
Two cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 C
With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.
Chapter 16 Solutions
EBK ORGANIC CHEMISTRY
Ch. 16.1 - Prob. 1PCh. 16.2 - Prob. 2PCh. 16.3 - Prob. 3PCh. 16.4 - Prob. 4PCh. 16.5 - Prob. 5PCh. 16.5 - Prob. 6PCh. 16.5 - Prob. 9PCh. 16.5 - Problem 18.9 Draw the product of each reaction
a....Ch. 16.5 - Prob. 11PCh. 16.5 - Prob. 12P
Ch. 16.5 - Prob. 13PCh. 16.6 - Prob. 14PCh. 16.6 - Problem 18.14 Draw all resonance structures for...Ch. 16.6 - Problem 18.15 Classify each substituent as...Ch. 16.7 - Prob. 17PCh. 16.9 - Prob. 22PCh. 16.10 - Problem 18.20 Draw the products of each...Ch. 16.10 - Prob. 24PCh. 16.11 - Problem 18.22 Draw the products formed when each...Ch. 16.12 - Problem 18.23 Devise a synthesis of each compound...Ch. 16.13 - Problem 18.24 Draw the products of each...Ch. 16.13 - Problem 18.25 Draw a stepwise mechanism for the...Ch. 16.13 - Problem 18.26 Draw the products of each...Ch. 16.13 - Prob. 30PCh. 16 - Prob. 37PCh. 16 - 18.35 What is the major product formed by an...Ch. 16 - 18.36 Draw the products formed when phenol is...Ch. 16 - Problem 18.37 Draw the products formed when each...Ch. 16 - 18.38 Draw the products of each reaction.
a. d....Ch. 16 - 18.39 What products are formed when benzene is...Ch. 16 - Prob. 49PCh. 16 - 18.47 For each of the following substituted...Ch. 16 - 18.48 Consider the tetracyclic aromatic compound...Ch. 16 - 18.49 For each N-substituted benzene, predict...Ch. 16 - Prob. 54PCh. 16 - 18.51 Using resonance structures, explain why a...Ch. 16 - Prob. 56PCh. 16 - 18.53 Rank the aryl halides in each group in order...Ch. 16 - Prob. 64PCh. 16 - Prob. 65PCh. 16 - Prob. 66PCh. 16 - 18.63 Synthesize each compound from benzene and...Ch. 16 - Problem 18.64 Synthesize each compound from...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY