
Conceptual Physical Science, Books a la Carte Edition; Modified Mastering Physics with Pearson eText -- ValuePack Access Card -- for Conceptual Physical Science (6th Edition)
6th Edition
ISBN: 9780134466927
Author: Paul G. Hewitt, Leslie A. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 45E
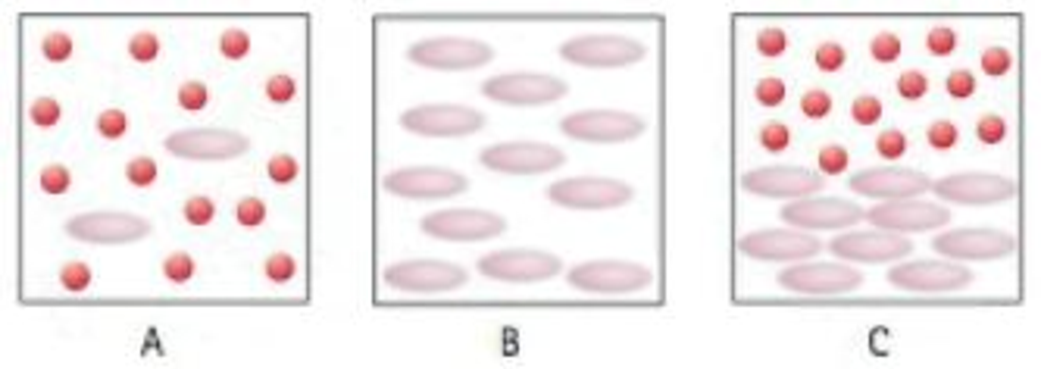
45. Which of these boxes best represents a suspension?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The cylindrical beam of a 12.7-mW laser is 0.920 cm in diameter. What is the rms value of the electric field?
V/m
Consider a rubber rod that has been rubbed with fur to give the rod a net negative charge, and a glass rod that has been rubbed with silk to give it a net positive charge. After being charged by contact by the fur and silk...?
a. Both rods have less mass
b. the rubber rod has more mass and the glass rod has less mass
c. both rods have more mass
d. the masses of both rods are unchanged
e. the rubber rod has less mass and the glass rod has mroe mass
8) 9)
Chapter 16 Solutions
Conceptual Physical Science, Books a la Carte Edition; Modified Mastering Physics with Pearson eText -- ValuePack Access Card -- for Conceptual Physical Science (6th Edition)
Ch. 16 - Prob. 1RCQCh. 16 - Prob. 2RCQCh. 16 - Prob. 3RCQCh. 16 - Prob. 4RCQCh. 16 - How is a solution different from a suspension?Ch. 16 - How can a solution be separated from a suspension?Ch. 16 - What happens to the volume of a sugar solution as...Ch. 16 - Prob. 8RCQCh. 16 - What does it mean to say that a solution is...Ch. 16 - Is concentration typically given with the volume...
Ch. 16 - Why does the solubility of a gas solute in a...Ch. 16 - Why do sugar crystals dissolve faster when...Ch. 16 - Is sugar a polar or nonpolar substance?Ch. 16 - Which portion of a soap molecule is nonpolar?Ch. 16 - What is the difference between a soap and a...Ch. 16 - Prob. 16RCQCh. 16 - Why are soap molecules so attracted to calcium and...Ch. 16 - Why is treated water sprayed into the air before...Ch. 16 - What are two ways in which people disinfect water...Ch. 16 - What naturally occurring element has been...Ch. 16 - Why can wastewater treatment requirements in...Ch. 16 - What is the first step in treating raw sewage?Ch. 16 - Prob. 23RCQCh. 16 - Prob. 30TASCh. 16 - Prob. 31TASCh. 16 - Prob. 32TASCh. 16 - How much sodium chloride, in grams, is needed to...Ch. 16 - If water is added to 1 mole of sodium chloride in...Ch. 16 - A student is told to use 20.0 g of sodium chloride...Ch. 16 - Rank the following solutions in order of...Ch. 16 - Rank the following compounds in order of...Ch. 16 - Prob. 38TARCh. 16 - How might you separate a mixture of sand and salt?...Ch. 16 - Mixtures can be separated into their components by...Ch. 16 - Why can't the elements of a compound be separated...Ch. 16 - Many dry cereals are fortified with iron, which is...Ch. 16 - The Chemist's Classification of Matter 43....Ch. 16 - Classify each of the following as an element,...Ch. 16 - 45. Which of these boxes best represents a...Ch. 16 - Prob. 46ECh. 16 - Prob. 47ECh. 16 - Prob. 48ECh. 16 - Which is more dense: air saturated with water...Ch. 16 - How many sugar molecules are there in a 2 M sugar...Ch. 16 - Prob. 51ECh. 16 - Which should weigh more: 100 mL of fresh water or...Ch. 16 - Explain why, for these three substances, the...Ch. 16 - The boiling point of 1,4-butanediol is 230C. Would...Ch. 16 - Based on atomic size, which would you expect to be...Ch. 16 - If nitrogen, N2, were pumped into your lungs at...Ch. 16 - Prob. 57ECh. 16 - Account for the observation that ethanol, C2H5OH,...Ch. 16 - At 10C, which is more concentrated: a saturated...Ch. 16 - Why is rain or snow called precipitation?Ch. 16 - Prob. 61ECh. 16 - Some bottled water is now advertised as containing...Ch. 16 - Two plastic bottles of fresh seltzer water are...Ch. 16 - Why can 500 mL of fresh water absorb more gaseous...Ch. 16 - Would you expect to find more dissolved oxygen in...Ch. 16 - Soaps, Detergents, and Hard Water Fatty acid...Ch. 16 - Fatty acid molecules can also align to form a...Ch. 16 - Prob. 68ECh. 16 - A scum forms on the surface of boiling hard water....Ch. 16 - Calcium and magnesium ions are more attracted to...Ch. 16 - Phosphate ions, PO43-, were once added to...Ch. 16 - Oils at the top of a tree have a higher...Ch. 16 - Why is distilling water so relatively expensive?Ch. 16 - What reverses with reverse osmosis?Ch. 16 - Why is it significantly less costly to purify...Ch. 16 - Prob. 76ECh. 16 - Many homeowners get their drinking; water piped up...Ch. 16 - Is the decomposition of food by bacteria in our...Ch. 16 - Where does most of the solid mass of raw sewage...Ch. 16 - Why is flushing a toilet with clean water from a...Ch. 16 - Why are people so willing to buy bottled water...Ch. 16 - It is possible to tow icebergs to coastal cities...Ch. 16 - Someone argues that he or she doesn't drink tap...Ch. 16 - Prob. 2RATCh. 16 - The air in your house is an example of a (a)...Ch. 16 - Half-frozen fruit punch is always sweeter than the...Ch. 16 - Why is sodium chloride, NaCl, insoluble in...Ch. 16 - Fish don't live very long in water that has just...Ch. 16 - Prob. 7RATCh. 16 - What is an advantage of using chlorine gas to...Ch. 16 - Why do red blood cells, which contain an aqueous...Ch. 16 - A stagnant pond smells worse than a babbling brook...
Additional Science Textbook Solutions
Find more solutions based on key concepts
11. The material that formed the earth was created in a supernova explosion approximately 6 billion years ago. ...
College Physics: A Strategic Approach (3rd Edition)
For the generic equilibrium HA(aq) ⇌ H + (aq) + A- (aq), which of these statements is true?
The equilibrium con...
Chemistry: The Central Science (14th Edition)
Name each of the following:
Organic Chemistry (8th Edition)
Examine the graph in Figure 6.3. Note that the growth rate increases slowly until the optimum is reached and th...
Microbiology with Diseases by Body System (5th Edition)
What is the anatomical position? Why is it important that you learn this position?
Anatomy & Physiology (6th Edition)
Name the components (including muscles) of the thoracic cage. List the contents of the thorax.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Lab 8 Part 3 PHET Wave Interface simulation. I am having trouble with this part of the lab.arrow_forwardMick and Rick are twins born on Earth in the year 2175. Rick grows up to be an Earth-bound robotics technician while Mick becomes an intergalactic astronaut. Mick leaves the Earth on his first space mission in the year 2200 and travels, according to his clock, for 10 years at a speed of 0.75c. Unfortunately, at this point in his journey, the structure of his ship undergoes mechanical breakdown and the ship explodes. How old is Rick when his brother dies?arrow_forwardHi, I have canceled, why did you charge me again?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
General Relativity: The Curvature of Spacetime; Author: Professor Dave Explains;https://www.youtube.com/watch?v=R7V3koyL7Mc;License: Standard YouTube License, CC-BY