
(a)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate two signals in
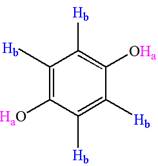
Explanation of Solution
The structure of the given compound is
The given compound has a plane of symmetry and thus has two chemically distinct protons indicated as
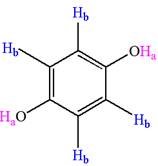
It is determined that the given molecule has two signals in
(b)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate five signals in
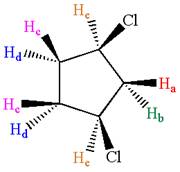
Explanation of Solution
The structure of the given compound is
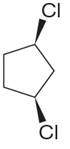
The protons cis to chlorine atoms and those trans to chlorine atoms are diastereotropic. Thus, the compound has five chemically distinct protons indicated as
It is determined that the given molecule has five signals in
(c)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate eight signals in
Explanation of Solution
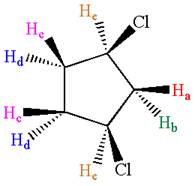
The structure of the given compound is
As the ring has two different substituents, all the protons are chemically non-equivalent. Thus, the compound has eight chemically distinct protons indicated as
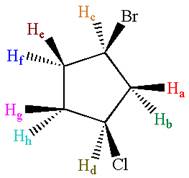
It is determined that the given molecule has eight signals in
(d)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate four signals in
Explanation of Solution
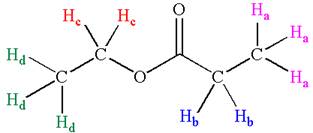
The structure of the given compound is
In the given compounds, both ethyl groups are in different chemical environment. One is attached to oxygen and another to carbonyl carbon. Thus the compound has four chemically distinct protons indicated as
It is determined that the given molecule has four signals in
(e)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate two signals in
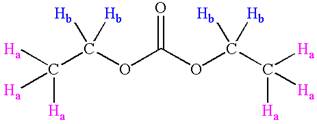
Explanation of Solution
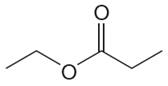
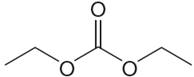
The structure of the given compound is
In the given compounds, both ethyl groups are in the same chemical environment. Thus, the compound has two chemically distinct protons indicated as
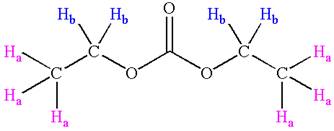
It is determined that the given molecule has two signals in
(f)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate three signals in
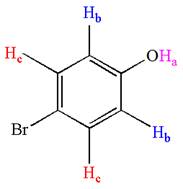
Explanation of Solution
The structure of the given compound is
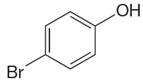
As the molecule is para disubstituted, it has a plane of symmetry passing through
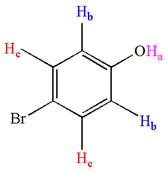
It is determined that the given molecule has three signals in
(g)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate five signals in
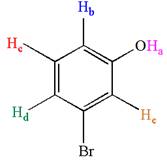
Explanation of Solution
The structure of the given compound is
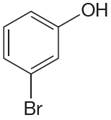
The molecule is meta disubstituted with different substituents and has no plane of symmetry; thus, all protons are chemically non-equivalent. Hence the compound has five chemically distinct protons indicated as
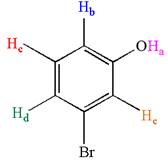
It is determined that the given molecule has five signals in
(h)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate five signals in
Explanation of Solution
The structure of the given compound is
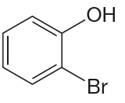
The molecule is ortho disubstituted with different substituents and has no plane of symmetry; thus, all protons are chemically non-equivalent. Hence the compound has five chemically distinct protons indicated as
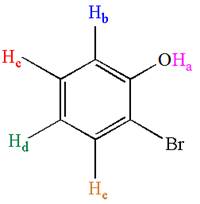
It is determined that the given molecule has five signals in
(i)
Interpretation:
The number of signals that would be generated in the
Concept introduction:
In
Answer to Problem 16.39P
The given molecule could generate six signals in
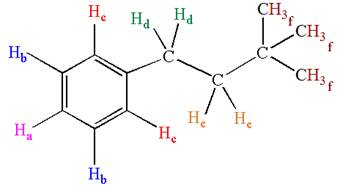
Explanation of Solution
The structure of the given compound is
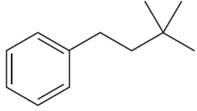
The molecule is monosubstituted benzene; thus, there are three types of aromatic protons. The three methyl protons are in same chemical environment; thus they are identical. Hence the compound has six chemically distinct protons indicated as

It is determined that the given molecule has six signals in
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry: Principles And Mechanisms
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forwardPredict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward
- 70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forwardShow your steps. Hopefully, you get everything correctly or a reasonable guess that is close to the correct answer.arrow_forward
- Please give it your best shot at answering this question.arrow_forwardLook the image attaarrow_forwardPart C: Communication (/9) 17. Compare and contrast the Thomson, Rutherford and Bohr models of the atom using the chart below. You can use words and/or diagrams in your answers. (9) What was the experiment that led to the model? Where is positive charge in the atom located in the model? Where are electrons located in the molecule? Thomson Model Rutherford Model Bohr Model 2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





