
Concept explainers
(a)
Interpretation:
The electron dot structure of
Concept Introduction:
Valence electrons are represented by dots placed around the chemical symbol. The valance electrons are placed around the elements (atoms) maximum electrons can fill around the atom. First fill the single electron around the atom and paired the electrons.
The valance electron for iodine atom is seven. Total valence electrons in triiodide anion are counted and electron dot structure for the anion is written. When iodine
(b)
Interpretation:
The
Concept Introduction:
Lewis definition:
A Lewis acid is a substance that can accept and share an electron pair.
A Lewis base is a substance that can donate and share an electron pair.
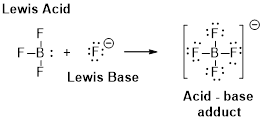
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
- ● Biological Macromolecules Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH2OH O H H H OH OH OH H H CH2OH H O OH H OH H H H H OH Is this a reducing sugar? Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Explanation Check O yes X O no ○ yes O no Uarrow_forwardThe aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forward
- ion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forward
- Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forward
- K Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





