
Conceptual Physics / MasteringPhysics (Book & Access Card)
12th Edition
ISBN: 9780321908605
Author: Paul G. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15, Problem 92RCQ
To determine
Whether the water will expand or contract at temperatures 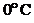 ,
, 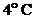 and
and 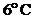
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Two concrete spans of a 234 m long bridge are placed end to end so that no room is allowed for expansion (Figure a). Each span therefore has a length of L0 = 117 m. If the temperature increases by 17.8 °C, what is the height y to which the spans rise when they buckle (Figure b)? (The coefficient of linear expansion of concrete is 1.20⋅10−51.20⋅10-5 °C−1.)
Monkey D. Luffy, from One Piece can inflate himself like a balloon to a size of 6.98 m3 by inhaling 1.74⋅10^26 molecules of air. If the air is at 20.9 ˚C, the pressure inside Luffy is 101277.062 Pa. kB=1.38⋅10^−23 J/K. The total internal energy of the gas inside Luffy is 1065333.93 J. How fast, on average, is the air molecules inside Luffy traveling at? The average mass of an air molecule (considering the various gasses involved) is 4.51 x 10^-26 kg.
The Dungeons & Dragons spell “Stinking Cloud” fills a 949 m^3 volume of air with a cloud of gas. The pressure of the gas is the same as the air, 101,325 Pa, and is at 29.2°C. There are 2.304x10^28 molecules of gas. What is the total internal energy of the gas?
Chapter 15 Solutions
Conceptual Physics / MasteringPhysics (Book & Access Card)
Ch. 15 - Prob. 1RCQCh. 15 - Prob. 2RCQCh. 15 - Prob. 3RCQCh. 15 - Prob. 4RCQCh. 15 - Prob. 5RCQCh. 15 - Prob. 6RCQCh. 15 - Prob. 7RCQCh. 15 - Prob. 8RCQCh. 15 - Prob. 9RCQCh. 15 - What role does temperature have in the direction...
Ch. 15 - Prob. 11RCQCh. 15 - Prob. 12RCQCh. 15 - Prob. 13RCQCh. 15 - Prob. 14RCQCh. 15 - Prob. 15RCQCh. 15 - Prob. 16RCQCh. 15 - Prob. 17RCQCh. 15 - Prob. 18RCQCh. 15 - Prob. 19RCQCh. 15 - According to the law of conservation of energy, if...Ch. 15 - Prob. 21RCQCh. 15 - Prob. 22RCQCh. 15 - Why does a bimetallic strip bend with changes in...Ch. 15 - Which generally expands more for an equal increase...Ch. 15 - Prob. 25RCQCh. 15 - Prob. 26RCQCh. 15 - Prob. 27RCQCh. 15 - Prob. 28RCQCh. 15 - Prob. 29RCQCh. 15 - Prob. 30RCQCh. 15 - Prob. 31RCQCh. 15 - Prob. 32RCQCh. 15 - The quantity of heat Q released or absorbed from a...Ch. 15 - 34. Use the same formula to show that 12,570...Ch. 15 - Prob. 35RCQCh. 15 - 36.Will Maynez burns a 0.6-g peanut beneath 50 g...Ch. 15 - 37. If you wish to warm 50 kg of water by 20°C for...Ch. 15 - 38. The specific heat capacity of steel is 450...Ch. 15 - Prob. 39RCQCh. 15 - Suppose that the 1.3-km main span of steel for...Ch. 15 - 41. Imagine a 40,000-km steel pipe that forms a...Ch. 15 - 42. Rank the magnitudes of these units of thermal...Ch. 15 - Prob. 43RCQCh. 15 - 44. How much the lengths of various substances...Ch. 15 - Prob. 45RCQCh. 15 - Prob. 46RCQCh. 15 - Prob. 47RCQCh. 15 - Prob. 48RCQCh. 15 - Prob. 49RCQCh. 15 - Why can’t you establish whether you are running a...Ch. 15 - Prob. 51RCQCh. 15 - Which has the greater amount of internal energy:...Ch. 15 - When a mercury thermometer is heated, the mercury...Ch. 15 - Prob. 54RCQCh. 15 - Prob. 55RCQCh. 15 - Prob. 56RCQCh. 15 - Prob. 57RCQCh. 15 - Prob. 58RCQCh. 15 - Prob. 59RCQCh. 15 - Which likely has the greater specific heat...Ch. 15 - If the specific heat capacity of water were less,...Ch. 15 - Prob. 62RCQCh. 15 - Prob. 63RCQCh. 15 - Prob. 64RCQCh. 15 - Prob. 65RCQCh. 15 - Prob. 66RCQCh. 15 - Prob. 67RCQCh. 15 - Prob. 68RCQCh. 15 - Prob. 69RCQCh. 15 - Prob. 70RCQCh. 15 - Prob. 71RCQCh. 15 - Prob. 72RCQCh. 15 - Prob. 73RCQCh. 15 - Prob. 74RCQCh. 15 - Prob. 75RCQCh. 15 - 76. Structural groaning noises are sometimes heard...Ch. 15 - Prob. 77RCQCh. 15 - Prob. 78RCQCh. 15 - Prob. 79RCQCh. 15 - Prob. 80RCQCh. 15 - Prob. 81RCQCh. 15 - Prob. 82RCQCh. 15 - Prob. 83RCQCh. 15 - Prob. 84RCQCh. 15 - Prob. 85RCQCh. 15 - Prob. 86RCQCh. 15 - Prob. 87RCQCh. 15 - What was the precise temperature at the bottom of...Ch. 15 - Prob. 89RCQCh. 15 - Prob. 90RCQCh. 15 - Prob. 91RCQCh. 15 - Prob. 92RCQCh. 15 - Prob. 93RCQCh. 15 - Prob. 94RCQCh. 15 - Prob. 95RCQCh. 15 - Prob. 96RCQCh. 15 - Prob. 97RCQCh. 15 - Heat added to a substance goes partly into the...Ch. 15 - 99. A metal ball is just able to pass through a...Ch. 15 - Prob. 100RCQCh. 15 - Prob. 101RCQCh. 15 - 102. Suppose that you cut a small gap in a metal...Ch. 15 - Prob. 103RCQCh. 15 - Prob. 104RCQCh. 15 - Prob. 105RCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The Fiero, which is 4.70 m long, starts at 10.0˚C while in the upper atmosphere but when it goes into space the temperature would be about -270.3˚C. How much should the steel siding of the Fiero shrink due to this temperature change? The coefficient of thermal linear expansion for steel is 11.0⋅10−6⋅10^-6 C-1arrow_forwardQuestion 3 of 17 L X L L T 0.5/ In the figure above, three uniform thin rods, each of length L, form an inverted U. The vertical rods each have a mass m; the horizontal rod has a mass 3m. NOTE: Express your answer in terms of the variables given. (a) What is the x coordinate of the system's center of mass? xcom L 2 (b) What is the y coordinate of the system's center of mass? Ycom 45 L X Q Search MD bp Narrow_forwardSketch the harmonic on graphing paper.arrow_forward
- Exercise 1: (a) Using the explicit formulae derived in the lectures for the (2j+1) × (2j + 1) repre- sentation matrices Dm'm, (J/h), derive the 3 × 3 matrices corresponding to the case j = 1. (b) Verify that they satisfy the so(3) Lie algebra commutation relation: [D(Î₁/ħ), D(Î₂/h)]m'm₁ = iƊm'm² (Ĵ3/h). (c) Prove the identity 3 Dm'm,(β) = Σ (D(Ρ)D(Ρ))m'¡m; · i=1arrow_forwardSketch the harmonic.arrow_forwardFor number 11 please sketch the harmonic on graphing paper.arrow_forward
- # E 94 20 13. Time a) What is the frequency of the above wave? b) What is the period? c) Highlight the second cycle d) Sketch the sine wave of the second harmonic of this wave % 7 & 5 6 7 8 * ∞ Y U 9 0 0 P 150arrow_forwardShow work using graphing paperarrow_forwardCan someone help me answer this physics 2 questions. Thank you.arrow_forward
- Four capacitors are connected as shown in the figure below. (Let C = 12.0 μF.) a C 3.00 με Hh. 6.00 με 20.0 με HE (a) Find the equivalent capacitance between points a and b. 5.92 HF (b) Calculate the charge on each capacitor, taking AV ab = 16.0 V. 20.0 uF capacitor 94.7 6.00 uF capacitor 67.6 32.14 3.00 µF capacitor capacitor C ☑ με με The 3 µF and 12.0 uF capacitors are in series and that combination is in parallel with the 6 μF capacitor. What quantity is the same for capacitors in parallel? μC 32.14 ☑ You are correct that the charge on this capacitor will be the same as the charge on the 3 μF capacitor. μCarrow_forwardIn the pivot assignment, we observed waves moving on a string stretched by hanging weights. We noticed that certain frequencies produced standing waves. One such situation is shown below: 0 ст Direct Measurement ©2015 Peter Bohacek I. 20 0 cm 10 20 30 40 50 60 70 80 90 100 Which Harmonic is this? Do NOT include units! What is the wavelength of this wave in cm with only no decimal places? If the speed of this wave is 2500 cm/s, what is the frequency of this harmonic (in Hz, with NO decimal places)?arrow_forwardFour capacitors are connected as shown in the figure below. (Let C = 12.0 µF.) A circuit consists of four capacitors. It begins at point a before the wire splits in two directions. On the upper split, there is a capacitor C followed by a 3.00 µF capacitor. On the lower split, there is a 6.00 µF capacitor. The two splits reconnect and are followed by a 20.0 µF capacitor, which is then followed by point b. (a) Find the equivalent capacitance between points a and b. µF(b) Calculate the charge on each capacitor, taking ΔVab = 16.0 V. 20.0 µF capacitor µC 6.00 µF capacitor µC 3.00 µF capacitor µC capacitor C µCarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON
A Level Physics – Ideal Gas Equation; Author: Atomi;https://www.youtube.com/watch?v=k0EFrmah7h0;License: Standard YouTube License, CC-BY