
Chemistry (Instructor's)
10th Edition
ISBN: 9781305957787
Author: ZUMDAHL
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 91AE
Tris(hydroxymethyl)aminomethane, commonly called TRIS or Trizma, is often used as a buffer in biochemical studies. Its buffering range is pH 7 to 9, and Kb is 1.19 × 10−6 for the aqueous reaction
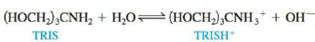
a. What is the optimal pH for TRIS buffers?
b. Calculate the ratio [TRIS]/[TRISH+] at pH = 7.00 and at pH = 9.00.
c. A buffer is prepared by diluting 50.0 g TRIS base and 65.0 g TRIS hydrochloride (written as TRISHCl) to a total volume of 2.0 L. What is the pH of this buffer?
What is the pH after 0.50 mL of 12 M HCl is added to a 200.0-mL portion of the buffer?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Seee the attached ima
Please see the attached image.
Please see the attached image.
Chapter 15 Solutions
Chemistry (Instructor's)
Ch. 15 - What is meant by the presence of a common ion? How...Ch. 15 - Define a buffer solution. What makes up a buffer...Ch. 15 - One of the most challenging parts of solving...Ch. 15 - A good buffer generally contains relatively equal...Ch. 15 - Draw the general titration curve for a strong acid...Ch. 15 - Instead of the titration of a strong acid by a...Ch. 15 - Sketch the titration curve for a weak acid...Ch. 15 - Sketch the titration curve for a weak base...Ch. 15 - What is an acidbase indicator? Define the...Ch. 15 - Why does an indicator change from its acid color...
Ch. 15 - What are the major species in solution after...Ch. 15 - A friend asks the following: Consider a buffered...Ch. 15 - Mixing together solutions of acetic acid and...Ch. 15 - Sketch two pH curves, one for the titration of a...Ch. 15 - Sketch a pH curve for the titration of a weak acid...Ch. 15 - You have a solution of the weak acid HA and add...Ch. 15 - You have a solution of the weak acid HA and add...Ch. 15 - The common ion effect for weak acids is to...Ch. 15 - Prob. 12QCh. 15 - A best buffer has about equal quantities of weak...Ch. 15 - Consider the following pH curves for 100.0 mL of...Ch. 15 - An acid is titrated with NaOH. The following...Ch. 15 - Consider the following four titrations. i. 100.0...Ch. 15 - Figure 14-4 shows the pH curves for the titrations...Ch. 15 - Acidbase indicators mark the end point of...Ch. 15 - Consider the titration of 100.0 mL of 0.10 M...Ch. 15 - Consider the following two acids: pKa1 = 2.98;...Ch. 15 - How many of the following are buffered solutions?...Ch. 15 - Which of the following can be classified as buffer...Ch. 15 - A certain buffer is made by dissolving NaHCO3 and...Ch. 15 - A buffer is prepared by dissolving HONH2 and...Ch. 15 - Calculate the pH of each of the following...Ch. 15 - Calculate the pH of each of the following...Ch. 15 - Compare the percent dissociation of the acid in...Ch. 15 - Compare the percent ionization of the base in...Ch. 15 - Calculate the pH after 0.020 mole of HCl is added...Ch. 15 - Calculate the pH after 0.020 mole of HCl is added...Ch. 15 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 15 - Calculate the pH after 0.020 mole of NaOH is added...Ch. 15 - Which of the solutions in Exercise 21 shows the...Ch. 15 - Prob. 34ECh. 15 - Calculate the pH of a solution that is 1.00 M HNO2...Ch. 15 - Calculate the pH of a solution that is 0.60 M HF...Ch. 15 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 15 - Calculate the pH after 0.10 mole of NaOH is added...Ch. 15 - Calculate the pH of each of the following buffered...Ch. 15 - Calculate the pH of each of the following buffered...Ch. 15 - Calculate the pH of a buffered solution prepared...Ch. 15 - A buffered solution is made by adding 50.0 g NH4Cl...Ch. 15 - Calculate the pH after 0.010 mole of gaseous HCl...Ch. 15 - Calculate the pH after 0.15 mole of solid NaOH is...Ch. 15 - Some K2SO3 and KHSO3 are dissolved in 250.0 mL of...Ch. 15 - An aqueous solution contains dissolved C6H5NH3Cl...Ch. 15 - Calculate the mass of sodium acetate that must be...Ch. 15 - What volumes of 0.50 M HNO2 and 0.50 M NaNO2 must...Ch. 15 - Consider a solution that contains both C5H5N and...Ch. 15 - Calculate the ratio [NH3]/[NH4+] in...Ch. 15 - Carbonate buffers are important in regulating the...Ch. 15 - When a person exercises, muscle contractions...Ch. 15 - Consider the acids in Table 13-2. Which acid would...Ch. 15 - Consider the bases in Table 13-3. Which base would...Ch. 15 - Calculate the pH of a solution that is 0.40 M...Ch. 15 - Calculate the pH of a solution that is 0.20 M HOCl...Ch. 15 - Which of the following mixtures would result in...Ch. 15 - Which of the following mixtures would result in a...Ch. 15 - What quantity (moles) of NaOH must be added to 1.0...Ch. 15 - Calculate the number of moles of HCl(g) that must...Ch. 15 - Consider the titration of a generic weak acid HA...Ch. 15 - Sketch the titration curve for the titration of a...Ch. 15 - Consider the titration of 40.0 mL of 0.200 M HClO4...Ch. 15 - Consider the titration of 80.0 mL of 0.100 M...Ch. 15 - Consider the titration of 100.0 mL of 0.200 M...Ch. 15 - Consider the titration of 100.0 mL of 0.100 M...Ch. 15 - Lactic acid is a common by-product of cellular...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Repeat the procedure in Exercise 61, but for the...Ch. 15 - Calculate the pH at the halfway point and at the...Ch. 15 - In the titration of 50.0 mL of 1.0 M methylamine,...Ch. 15 - You have 75.0 mL of 0.10 M HA. After adding 30.0...Ch. 15 - A student dissolves 0.0100 mole of an unknown weak...Ch. 15 - Two drops of indicator HIn (Ka = 1.0 109), where...Ch. 15 - Methyl red has the following structure: It...Ch. 15 - Potassium hydrogen phthalate, known as KHP (molar...Ch. 15 - A certain indicator HIn has a pKa of 3.00 and a...Ch. 15 - Which of the indicators in Fig. 14-8 could be used...Ch. 15 - Prob. 80ECh. 15 - Which of the indicators in Fig. 14-8 could be used...Ch. 15 - Prob. 82ECh. 15 - Estimate the pH of a solution in which bromcresol...Ch. 15 - Estimate the pH of a solution in which crystal...Ch. 15 - A solution has a pH of 7.0. What would be the...Ch. 15 - A solution has a pH of 4.5. What would be the...Ch. 15 - When a diprotic acid, H2A. is titrated with NaOH,...Ch. 15 - Consider die titration of 50.0 mL of 0.10 M H3A...Ch. 15 - Derive an equation analogous to the...Ch. 15 - a. Calculate the pH of a buffered solution that is...Ch. 15 - Tris(hydroxymethyl)aminomethane, commonly called...Ch. 15 - You make 1.00 L of a buffered solution (pH = 4.00)...Ch. 15 - You have the following reagents on hand: Solids...Ch. 15 - Prob. 94AECh. 15 - Phosphate buffers are important in regulating the...Ch. 15 - When a diprotic acid, H2A, is titrated with NaOH,...Ch. 15 - Consider the blood buffer system discussed in the...Ch. 15 - What quantity (moles) of HCl(g) must be added to...Ch. 15 - Prob. 99AECh. 15 - The following plot shows the pH curves for the...Ch. 15 - Calculate the volume of 1.50 102 M NaOH that must...Ch. 15 - Prob. 102AECh. 15 - A certain acetic acid solution has pH = 2.68....Ch. 15 - A 0.210-g sample of an acid (molar mass = 192...Ch. 15 - The active ingredient in aspirin is...Ch. 15 - One method for determining the purity of aspirin...Ch. 15 - A student intends to titrate a solution of a weak...Ch. 15 - A student titrates an unknown weak acid, HA, to a...Ch. 15 - A sample of a certain monoprotic weak acid was...Ch. 15 - The pigment cyanidin aglycone is one of the...Ch. 15 - Consider 1.0 L of a solution that is 0.85 M HOC6H5...Ch. 15 - What concentration of NH4Cl is necessary to buffer...Ch. 15 - Consider the following acids and bases: HCO2H Ka =...Ch. 15 - Consider a buffered solution containing CH3NH3Cl...Ch. 15 - Consider the titration of 150.0 mL of 0.100 M HI...Ch. 15 - Consider the titration of 100.0 mL of 0.100 M HCN...Ch. 15 - Consider the titration of 100.0 mL of 0.200 M...Ch. 15 - Consider the following four titrations (iiv): i....Ch. 15 - Another way to treat data from a pH titration is...Ch. 15 - A buffer is made using 45.0 mL of 0.750 M HC3H5O2...Ch. 15 - A 0.400-M solution of ammonia was titrated with...Ch. 15 - What volume of 0.0100 M NaOH must be added to 1.00...Ch. 15 - Consider a solution formed by mixing 50.0 mL of...Ch. 15 - Cacodylic acid, (CH3)2AsO2H, is a toxic compound...Ch. 15 - The titration of Na2CO3 with HCl bas the following...Ch. 15 - Consider the titration curve in Exercise 115 for...Ch. 15 - A few drops of each of the indicators shown in the...Ch. 15 - Malonic acid (HO2CCH2CO2H) is a diprotic acid. In...Ch. 15 - A buffer solution is prepared by mixing 75.0 mL of...Ch. 15 - A 10.00-g sample of the ionic compound NaA, where...Ch. 15 - Calculate the pH of a solution prepared by mixing...Ch. 15 - Consider a solution prepared by mixing the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY