
Conceptual Physical Science Plus Mastering Physics with Pearson eText -- Access Card Package (6th Edition)
6th Edition
ISBN: 9780134060484
Author: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 15, Problem 77E
To determine
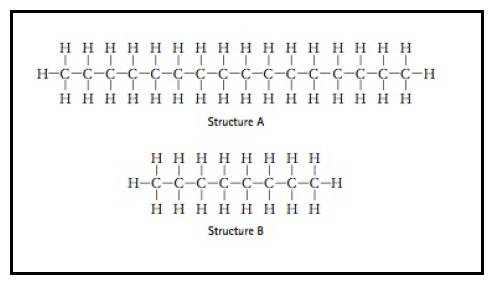
Of the two structures given below, the one which belong to the structure of gasoline and the other one that belong to structure of motor oil.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank you
A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).
What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V)
ammeter
I =
Chapter 15 Solutions
Conceptual Physical Science Plus Mastering Physics with Pearson eText -- Access Card Package (6th Edition)
Ch. 15 - How many electrons can occupy the first shell? How...Ch. 15 - Which electrons are represented by an electron-dot...Ch. 15 - Prob. 3RCQCh. 15 - How does an ion differ from an atom?Ch. 15 - To become a negative ion, does an atom lose or...Ch. 15 - Why does the fluorine atom tend to gain only one...Ch. 15 - Prob. 7RCQCh. 15 - Suppose an oxygen atom gains two electrons to...Ch. 15 - Prob. 9RCQCh. 15 - Do metals more readily gain or lose electrons?
Ch. 15 - What is an alloy?Ch. 15 - What is a native metal?Ch. 15 - Prob. 13RCQCh. 15 - Prob. 14RCQCh. 15 - Within a neutral molecule, how many covalent bonds...Ch. 15 - Prob. 16RCQCh. 15 - Prob. 17RCQCh. 15 - Prob. 18RCQCh. 15 - Prob. 19RCQCh. 15 - How can a molecule be nonpolar when it consists of...Ch. 15 - Why do nonpolar substances boil at relatively low...Ch. 15 - Which is more symmetrical: a polar molecule or a...Ch. 15 - Why dont oil and water mix?Ch. 15 - Prob. 24RCQCh. 15 - Which is stronger: the ion-dipole attraction or...Ch. 15 - What is a hydrogen bond?Ch. 15 - Are induced dipoles permanent?Ch. 15 - Prob. 31TASCh. 15 - What is the electric charge on the calcium ion in...Ch. 15 - Prob. 33TASCh. 15 - Prob. 34TASCh. 15 - Rank these bonds in order of increasing polarity:...Ch. 15 - Prob. 36TARCh. 15 - Prob. 37TARCh. 15 - Prob. 38TARCh. 15 - Prob. 39ECh. 15 - Prob. 40ECh. 15 - How many more electrons can fit within the valence...Ch. 15 - Prob. 42ECh. 15 - What happens when hydrogens electron gets close to...Ch. 15 - Prob. 44ECh. 15 - Why does an atom with few valence electrons tend...Ch. 15 - Why is it so easy for a magnesium atom to lose two...Ch. 15 - Why doesnt the neon atom tend to lose or gain any...Ch. 15 - Why does an atom with many valence electrons tend...Ch. 15 - Sulfuric acid, H2SO4, loses two protons to form...Ch. 15 - Prob. 50ECh. 15 - Which should be more difficult to pull apart: a...Ch. 15 - Prob. 52ECh. 15 - Given that the total number of atoms on our planet...Ch. 15 - An artist wants to create a metal sculpture using...Ch. 15 - Two fluorine atoms join together to form a...Ch. 15 - How are metallic bonds similar to ionic bonds? How...Ch. 15 - What drives an atom to form a covalent bond: its...Ch. 15 - Atoms of nonmetallic elements form covalent bonds,...Ch. 15 - Prob. 59ECh. 15 - Prob. 60ECh. 15 - Write the electron-dot structure for the covalent...Ch. 15 - Prob. 62ECh. 15 - In each molecule, which atom carries the greater...Ch. 15 - Which is more polar: a sulfur-bromine (S-Br) bond...Ch. 15 - True or False: The greater the nuclear charge of...Ch. 15 - True or False: The more shells in an atom, the...Ch. 15 - Water, H2O, and methane, CH4, have about the same...Ch. 15 - In the figure on the next page, the molecule from...Ch. 15 - Prob. 69ECh. 15 - Three kids sitting equally apart around a table...Ch. 15 - Which is stronger: the covalent bond that holds...Ch. 15 - The charges with sodium chloride are all...Ch. 15 - Prob. 73ECh. 15 - Prob. 74ECh. 15 - Prob. 75ECh. 15 - A thin stream of water is pulled to a rubber...Ch. 15 - Prob. 77ECh. 15 - Prob. 1RATCh. 15 - Prob. 2RATCh. 15 - Which would you expect to have a higher melting...Ch. 15 - Why are ores so valuable? (a) They are sources of...Ch. 15 - In terms of the periodic table, is there an abrupt...Ch. 15 - A hydrogen atom does not form more than one...Ch. 15 - When nitrogen and fluorine combine to form a...Ch. 15 - Prob. 8RATCh. 15 - Prob. 9RATCh. 15 - Iodine, I2, has a higher melting point than...
Knowledge Booster
Similar questions
- simple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forwardAn L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forward
- A long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forwardDiscuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forward
- Explain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forwardA 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forward
- For each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forwardWhen violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forwardTwo complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning


Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning